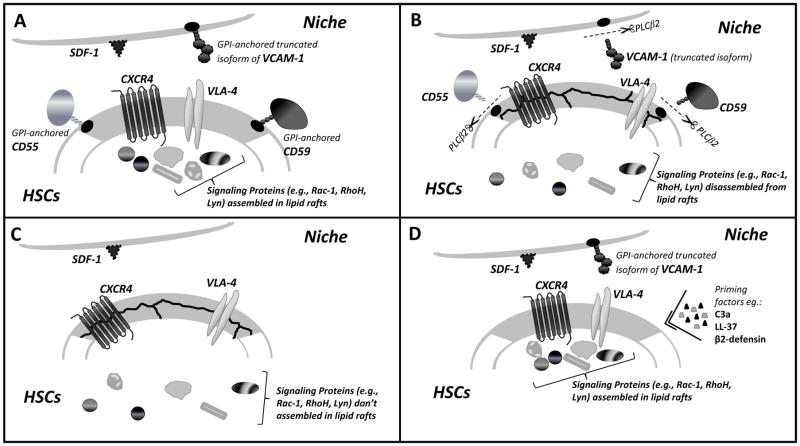
Figure 1.
Panel A. The role of lipid rafts in retention of HSPCs in stem cell niches. Membrane lipid rafts, shown as gray areas in the cell membrane, assemble with several cell surface receptors involved in retention of HSPCs in bone marrow niches (e.g., CXCR4 and VLA-4) or inhibition of the complement cascade (CD55 and CD59). As shown, CD55, CD59 and the murine truncated isoform of VCAM-1 are GPI-anchored proteins. While the role of lipid rafts is well described for cell membranes present in HSPCs, more studies are needed to determine whether bone marrow-retention ligands for HSPCs in stem cell niches, such as SDF-1 and VCAM-1, are also concentrated in lipid rafts in cells lining stem cell niches (e.g., osteoblasts, endothelial cells, and CAR cells). Panels B and C. Disassembly of lipid rafts and the egress of HSPCs into blood. Lipid raft disassembly results in the weakening of CXCR4–SDF-1 and VLA-4–VCAM-1 retention signals for HSPCs in bone marrow stem cell niches and facilitates mobilization. This effect is observed during pharmacological mobilization due to the release of phospholipase Cβ2 from granulocytes and monocytes (panel B) or in paroxysmal nocturnal hemoglobinuria (PNH) patients due to lack of expression of GPI-A and thus lack of GPI-anchored proteins (panel C). Disassembly of lipid rafts in HSPCs, as shown, weakens the interaction of both receptors with downstream signaling molecules involved in bone marrow retention. While in the case of HSPC mobilization a pivotal role is played by enzymatic digestion of GPI-anchored proteins by PLC-Cβ2, PNH results from an acquired mutation for the gene encoding GPI-A. Panel D. CXCR4 and VLA-4 inclusion in lipid rafts facilitates homing of HSPCs. Incorporation of the crucial BM homing receptors CXCR4 and VLA-4 into lipid rafts may be enhanced by exposure of HSPCs to certain molecules related to innate immunity, such as the C3a complement cascade cleavage fragment, LL-37 (cathelicidin), or β2-defensin, released from bone marrow stromal cells after radiochemotherapy-induced conditioning for transplantation. Incorporation of the CXCR4 receptor into lipid rafts sensitizes the responsiveness of HSPCs to an SDF-1 gradient. Colocalization of VLA-4 in membrane lipid rafts also plays an important role in tethering HSPCs to VCAM-1 in BM niches. As shown, lipid rafts facilitate the interaction of both receptors expressed on HSPCs with several downstream signaling molecules involved in migration, homing, and cell survival.
For simplicity, the interaction of lipid raft-associated c-kit receptor, which inhibits apoptosis and regulates proliferation of HSPCs, with its ligand, membrane-bound or soluble SCF, is not shown in the figure.