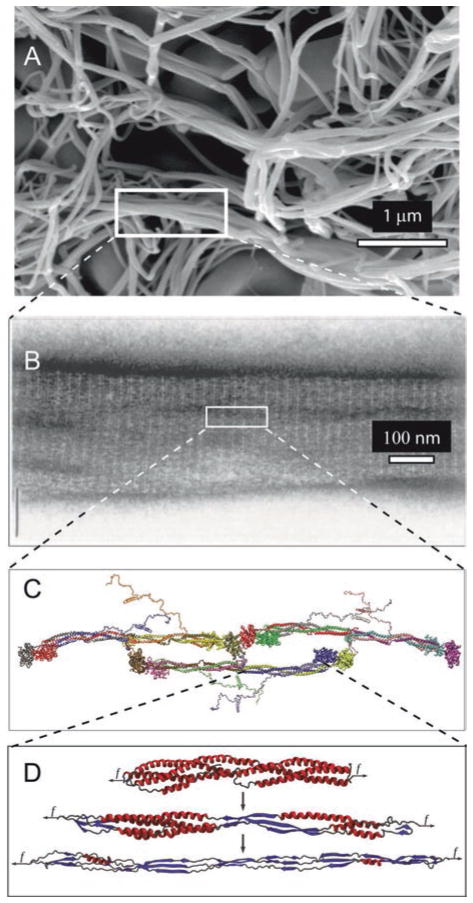
Fig. 13.10.
Unfolding of the coiled-coils of fibrin. (a). scanning electron micrograph of a fibrin clot, with a box enclosing part of a fiber. (b). A transmission electron micrograph of a negatively contrasted fibrin fiber showing the ultrastructure, with the 22.5 nm repeat arising from the half-staggering of 45-nm molecules. (c). A fibrin trimer from X-ray crystallographic data and molecular dynamics simulations of regions not present in the crystal structure. (d). Fibrin α-helical coiled coils undergoing a forced transition from α-helix to β-sheet. The mechanical transition from α-helical coiled coils to β-sheets in the fibrin(ogen) molecule was characterized using molecular dynamics simulations of their forced elongation and theoretical modeling (Adapted with permission from Zhmurov et al. 2012. Copyright 2012 American Chemical Society)