Abstract
Objective
To evaluate the safety and efficacy of dexmedetomidine (Dex) to prevent emergence agitation (EA) and delirium (ED) in children undergoing laparoscopic hernia repair under general anesthesia.
Methods
100 children (1–5 years, 10–25 kg) were randomized into four groups: controls (saline) and intravenous Dex at 0.25, 0.5, and 1.0 µg/kg (D1, D2, D3, respectively). Dex/saline infusion was started following anesthesia. EA and ED were evaluated on a 5-point scale.
Results
For the C, D1, D2, and D3 groups, respectively, EA frequencies were 45.8%, 30.4%, 12%, 4%; ED frequencies 29.1%, 13%, 4%, 4%; CHIPPS scores 8, 6, 3, 3; sevoflurane doses from 13.2 ± 3.4 (controls) to 9.4 ± 3.5 ml (D3). Intervals until mask removal/spontaneous eye opening were significantly longer for D2 and D3 than controls. PACU stay was longer for D3.
Conclusions
There was significantly less postoperative EA and pain, with less sevoflurane required, using Dex.
Keywords: Dexmedetomidine, emergency agitation, emergency delirium, laparoscopic hernia repair, general anesthesia, children
Introduction
In recent years, the number of children subjected to laparoscopic hernia repair under general anesthesia has increased because of the development of minimally invasive approaches and anesthesia techniques. Nevertheless, emergence agitation (EA) and emergence delirium (ED) remain challenging for anesthesiologists. EA is a mental state in which there is a lack of connection between consciousness and the patient’s behavior, which is characterized by excitement, irritability, disorientation, and inappropriate behavior. In contrast, ED is a transient disturbance of consciousness, which could be complicated by agitation, hallucination, and incoherent thoughts.1 It is characterized by disturbances in perception of, or even noticing, the surrounding environment associated with disorientation and perceptual changes. EA and ED are usually transient but are distressing for parents and staff. In some cases, they result in self-injury and the need for restraints.1 The frequency of EA and ED depends on the type of anesthetic agents used.2
Dexmedetomidine (Dexdor™) is a novel, highly selective, α2-adrenergic receptor (α2AR) agonist that has been widely used for adult anesthesia and as a sedative in intensive care units. Some previous studies suggested that dexmedetomidine (Dex) is safe in children.3–5 With its hypnotic, analgesic, sedative, and anxiolytic effects,6 it has been shown to improve intraoperative hemodynamic stability, minimize responses to stimuli, and reduce the need for other anesthetic agents.7 Previous studies, however, have reported conflicting results on the effects of Dex on EA and ED.8,9 Many of these studies in pediatric populations used Dex during surgery.3,4
The aim of the present preliminary study was to determine a safe, effective dose of preoperative Dex that would prevent postoperative EA and ED in children undergoing laparoscopic hernia repair under general sevoflurane anesthesia.
Patients and methods
Patients
Consecutive children with an inguinal hernia undergoing high ligation of the hernial sac via laparoscopy at our hospitals between January and March 2014 were enrolled in this preliminary prospective study. Inclusion criteria were (1) grade I–II in the American Society of Anesthesiologists classification; (2) preoperative diagnosis of unilateral inguinal hernia; (3) 1–5 years old; (4) weight ≥10 kg; (5) surgery conducted by the same surgeon. Exclusion criteria were (1) history of respiratory tract infection 1 week preoperatively; (2) preoperative liver and/or kidney dysfunction; (3) any congenital malformation or acquired disease that could increase the risks of anesthesia and the dose of anesthetics (such as, but not limited to, congenital heart disease, hydronephrosis, nutrition dysplasia); (4) mental abnormalities; (5) long-term use of sedative or analgesic drugs.
The ethics committees of our hospitals approved this study. Parents of the children were well informed before the anesthesia was started and provided written informed consent.
Study design
Children were randomized 1:1 to one of four groups (n = 25/group) using a computer-generated random numbers table: a control group (group C, which received saline) and three treatment groups, which were given different Dex doses (D1, D2, and D3 groups receiving doses of 0.25, 0.5, and 1.0 µg/kg, respectively). Randomization was performed upon arriving in the surgical ward. Saline or Dex was prepared by the attending anesthesiologist according to randomization. All other study personnel and observers were blinded to the study grouping.
Two anesthesiologists were involved in the study. All anesthetics were prepared behind drapes and administered by the first anesthesiologist according to randomization. All variables were recorded by the second anesthesiologist who was blinded to the randomization.
Experimental methods
Each child, accompanied by the parents, was admitted to the ward on the afternoon of the day before surgery. A indwelling catheter was inserted under local anesthesia for fluid infusion during the fasting period. Children were fasted for 6 h, and those aged 6 months to 3 years were forbidden to drink for 3 h preoperatively to decrease the risk of regurgitation and aspiration when intra-abdominal pressure was increased for laparoscopy. Because parents were not allowed on the surgical ward, anesthesia was induced in a dedicated room where the parents could be present to reassure their child. The child was carried to the operating room after loss of consciousness.
All anesthesia procedures were performed by the same attending anesthesiologist using intravenous injections of phencyclidine 0.01 mg/kg (Sernyl™, lot #130423; Chengdu List Pharmaceutical Co., Ltd., Chengdu, China), midazolam 0.05 mg/kg (Versed™, lot #20130806; Jiangsu Nhwa Pharmaceutical Co., Ltd., Jiangsu, China), fentanyl 2.5 µg/kg (Sufenta™, lot #1131214; Yichang Humanwell Pharmaceutical Co., Ltd., Yichang, China), propofol 2.5 mg/kg (Diprivan™, lot #KJ985; AstraZeneca, London, UK), and cis-atracurium 0.08 mg/kg (Nimbex™, lot #A20130811; Dongying (Jiangsu) Pharmaceutical Co., Ltd., Shanghai, China). In our center, phencyclidine is used to inhibit respiratory secretions during surgery.
A laryngeal mask airway was established for mechanical ventilation 30 s after the Observer’s Assessment of Alertness Sedation score reached grade VI according to the anesthesia machine (OHMEDA-S Avance; GE Healthcare, Waukesha, WI, USA) with the following settings: pressure 9–18 cmH2O; respiratory rate 18–25 breaths/min; respiratory ratio 1.0:1.5–2.0; end-tidal carbon dioxide partial pressure (PETCO2) of 35–40 mmHg].
Anesthesia was maintained with inhalation of oxygen-sevoflurane 3%, 2 L/min (Sojourn™, lot #44101; Maruishi Pharmaceutical Co., Ltd., Osaka, Japan) using a sevoflurane vaporizer (Abbott Laboratories, Abbott Parks, IL, USA). Anal acetaminophen suppository 7 mg/kg (lot #130709; Dongxin Pharmaceutical Co., Ltd., Hubei, China) was used for analgesia. Sequentially, constant intravenous infusion of normal saline (2 ml) or Dex (0.2 mg in 2 ml) (Dexdor™, lot #20130902; Jiangsu Nhwa Pharmaceutical Co., Ltd., Xuzhou, China) was administered within 10 min prior to surgery using a micro-pump (Beijing Slgo Medical Technology Co., Ltd., Beijing, China). The Dex solutions were prepared by the attending anesthesiologist using normal saline.
The child’s electrocardiogram, peripheral oxyhemoglobin saturation (SpO2), mean arterial pressures (MAP), and PETCO2 were monitored. Lactated Ringer’s solution (10 ml/kg/h) was administered intraoperatively for fluid maintenance. The heart rate (HR) and MAP between anesthesia induction and prior to establishing the laryngeal mask airway were used as baselines, and the intraoperative HR and MAP were controlled within a 30% variation around the baseline levels. The end-tidal sevoflurane concentration/minimum alveolar concentration (SEVET/MAC) was maintained at 0.6–1.5 by adjusting the sevoflurane concentration.
The drugs were stopped 3–5 min postoperatively, and the child was transferred to the postanesthesia care unit (PACU). The laryngeal mask was removed in the operating room when spontaneous breathing was recovered and PETCO2 was <45 mmHg. The transfer time to the PACU took <1 min in all cases.
Endpoints
The primary endpoints were the frequencies of EA and ED 2 h postoperatively according to a 5-point scale (Table 1).10,11 Delirium was defined as an agitation score of ≥4 for ≥5 min despite all efforts taken by the parents to calm the child.
Table 1.
Five-point scale for emergence agitation and delirium11
| Factors | Score |
|---|---|
| Sleeping | 1 |
| Awake and calm | 2 |
| Irritable and crying | 3 |
| Inconsolable crying | 4 |
| Severe restlessness and disorientation | 5 |
The secondary endpoints included Children’s and Infants’ Postoperative Pain Scale (CHIPPS) scores (Table 2) within 2 h postoperatively, intraoperative doses of sevoflurane, duration of the surgery, time between the end of anesthesia and laryngeal mask removal (TE), time to spontaneous eye opening (TA), and duration of PACU stay (TP) as well as the HR, MAP, SPO2, and PETCO2 values recorded at various time points—i.e., prior to Dex/saline infusion (T1), surgery initiation (T2), full insufflation (T3), end of surgery (T4), after laryngeal mask removal (T5), after transfer to the PACU (T6), and discharge from the PACU (T7)]. The presence or absence of anesthesia adverse effects such as laryngospasm, apnea, arrhythmia, hypotension, hypertension, and vomiting were recorded.12
Table 2.
Children and Infants Postoperative Pain Scale (CHIPPS)13
| Item | Criteria and score for each item |
||
|---|---|---|---|
| Score 0 | Score 1 | Score 2 | |
| Crying | None | Groaning | Screaming |
| Facial expression | At ease or smiling | Pouting | Expression of pain |
| Trunk posture | Middle | Tossing and turning | Buckling and stiffness |
| Lower limb posture | Relaxed or straightened | Kicking around | Tightened in both legs |
| Restlessness | None | Consolable | Irritated and disturbed |
Middle: Neutral, the stillness of the body, in a relaxed posture, no activity.
All five scores are added together. During the postoperative period, total scores between 0 and 3 indicated a painless state. A score of ≥4 indicated the need for analgesic drugs.
Assessment of emergence agitation and delirium
The appearance of EA and ED was evaluated every 5 min using a 5-point scale (Table 1).10,11 The analgesic effect was assessed using the CHIPPS scale (Table 2)13 applied by the same PACU physician who was blinded to the grouping. With the 5-point scale (Table 1), EA was defined as a score of ≥3 and ED as a score of ≥4 for ≥5 min.14 Scoring criteria of the 5-point scale11 were 0 = falling asleep peacefully; 1 = quiet; 2 = easy to console; 3 = difficult to console with moderate agitation; 4 = combative, excited, and disorientated.
Intravenous midazolam 0.05–0.1 mg/kg was administered to children exhibiting EA. If the symptoms were not relieved, intravenous sufentanyl 0.2 µg/kg was given. The child was transferred from the PACU to the general ward when the Steward score was ≥ 415 (or the modified Aldrete score was ≥916). The CHIPPS score continued to be monitored for 2 h postoperatively. Only the highest scores were used in the statistical analyses.
Statistical analysis
Continuous variables were tested using the normality and homogeneity of variance tests. Normally distributed continuous variables are expressed as means ± standard deviation and were analyzed using analysis of variance (ANOVA) with the Bonferroni test as the post hoc analysis. Repeated measures were analyzed by repeated measures ANOVA. Non-normally distributed variables are expressed as medians (interquartile range) and were analyzed using the Kruskal–Wallis test and the post hoc Mann–Whitney U test. Categorical variables are presented as frequencies and were analyzed using the χ2 or Fisher’s exact test, as appropriate. SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Values of P < 0.05 were considered to indicate statistical significance.
Results
Patients’ characteristics
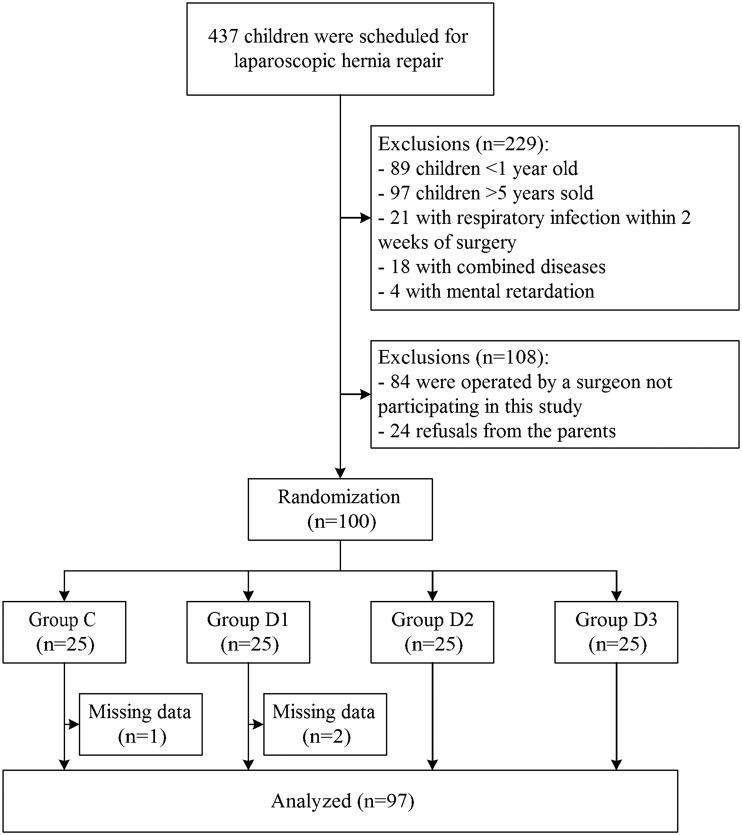
Figure 1 shows the patients’ flow chart: 437 patients were considered for recruitment, 337 were excluded for various reasons, and 3 had to be excluded because of an incomplete data set. Thus, 97 patients were finally included in the study.
Figure 1.
Patients’ flowchart.
Table 3 presents the baseline characteristics of the included patients. There were no significant differences in age, weight, sex, or duration of surgery (all P > 0.05). Compared with the controls, TE and TA were significantly longer in group D2, and the TE, TA, and TP were significantly longer in group D3 (all P < 0.05). No significant differences were found in any index between group D1 and the controls (all P > 0.05).
Table 3.
Characteristics of the children
| Variable | Group C | Group D1 | Group D2 | Group D3 | P |
|---|---|---|---|---|---|
| No. in the group | 24 | 23 | 25 | 25 | |
| Age (months) | 24.1 ± 11.1 | 26.4 ± 10.1 | 25.8 ± 10.1 | 26.1 ± 9.7 | 0.481 |
| Weight (kg) | 12.9 ± 2.7 | 13.4 ± 3.2 | 13.2 ± 2.8 | 13.4 ± 3.1 | 0.857 |
| Sex (male/female) | 16/8 | 17/5 | 18/7 | 19/5 | 0.901 |
| Hernia (single/double) | 15/9 | 16/7 | 15/10 | 14/11 | 0.821 |
| TS (min) | 18.0 ± 7.0 | 17.2 ± 6.3 | 17.6 ± 4.6 | 16.4 ± 4.8 | 0.850 |
| TE (min) | 2.7 ± 0.7 | 2.7 ± 0.8 | 4.4 ± 1.0a | 5.0 ± 1.1a | < 0.001 |
| TA (min) | 8.8 ± 1.4 | 9.4 ± 1.4 | 11.2 ± 2.8a | 13.8 ± 2.6a | <0.001 |
| TP (min) | 15.7 ± 1.7 | 15.7 ± 1.7 | 17.2 ± 3.6 | 17.8 ± 2.9a | 0.031 |
TS: time of surgery; TE: time between the end of anesthesia and laryngeal mask removal; TA: time to spontaneous eye opening; TP: time of stay in the postanesthesia care unit.
P < 0.05 vs. group C (analysis of variance with the post hoc Bonferroni test).
Postoperative agitation scale and CHIPPS scale
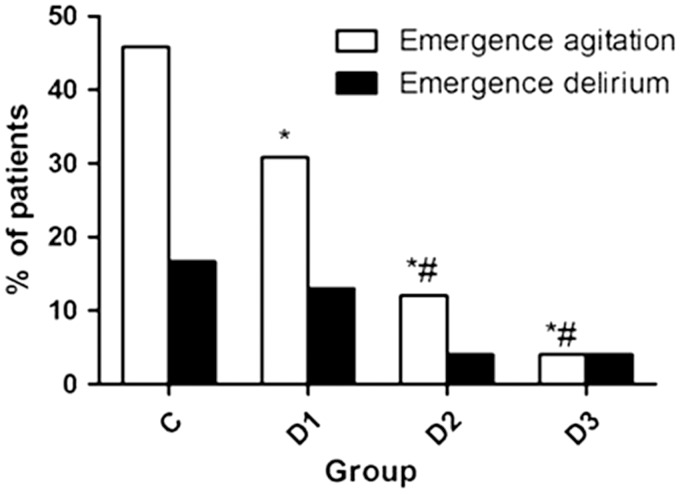
Table 4 presents the EA, ED, and pain scores of the children. Dex reduced the pain of the children, as shown by the CHIPPS score (P < 0.001), with Dex at 0.5 and 1.0 µg/kg having better efficacy than at 0.25 µg/kg. The frequency of EA shows a declining trend with increasing doses of Dex (P = 0.001) (Figure 2).
Table 4.
Pain and agitation scores 2 h postoperatively
| Parameter | Group C (n = 24) | Group D1 (n = 23) | Group D2 (n = 25) | Group D3 (n = 25) | P |
|---|---|---|---|---|---|
| CHIPPS scale | 8 (6–9)a | 6 (5–9)a | 3 (2–4)b | 3 (2–4)b | <0.001 |
| 5-Point scale | 3 (3–4)a | 3 (2–4)a | 2 (1–2)b | 1 (1–2)b | <0.001 |
| EA frequency | 11 (45.8%) | 7 (30.4%)a | 3 (12.0%)ab | 1 (4.0%)ab | 0.001 |
| ED frequency | 7 (29.1%) | 3 (13.0%) | 1 (4.0%) | 1 (4.0%) | 0.341 |
Scores are presented as the median (IQR) and were analyzed with the Kruskal–Wallis test and the post hoc Mann–Whitney U test.
Categorical variables are presented as proportions and were analyzed with Fisher’s exact test.
Dex: dexmedetomidine; CHIPPS: Children and Infants Postoperative Pain Scale; EA: emergence agitation; ED: emergence delirium.
P < 0.05 vs. controls
P < 0.05 vs. Dex 0.25
Figure 2.
Occurrence of emergence agitation (EA) and delirium (ED). *P < 0.05 vs. controls. #P < 0.05 vs. dexmedetomidine 0.25 µg/kg.
Sevoflurane consumption
Intraoperative consumption of sevoflurane decreased with the increasing dose of Dex, especially in groups D2 and D3 (P < 0.05). No significant differences were found between group D1 and the controls or between groups D2 and D3 (P > 0.05) (Table 5).
Table 5.
Comparison of sevoflurane consumption in the four groups
| Group | No. of patients | Sevoflurane consumption (ml) |
|---|---|---|
| C | 24 | 13.2 ± 3.4 |
| D1 | 23 | 12.2 ± 3.6# |
| D2 | 25 | 9.5 ± 4.1* |
| D3 | 25 | 9.4 ± 3.5** |
P < 0.05 vs. group C.
P < 0.01 vs. group C.
P < 0.05 vs. group D2.
Intraoperative hemodynamic changes in the children
MAP and HR of the patients in the controls showed increasing trends after the surgery had begun. These parameters in groups D1 and D2 showed decreasing trends after the Dex injection, although these changes were within 30% of the baseline levels (all P > 0.05). A transient, but significant, increase in blood pressure was observed in group D3 at the initiation of surgery compared with the baseline (P < 0.05). The MAP and HR gradually recovered after the Dex infusion was terminated. No significant differences were found for MAP or HR in the group D2 patients at any time point (Table 6).
Table 6.
Variations in the HR and MAP in patients of the four groups
| Parameter, by group | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|
| Heart rate (bpm) | |||||||
| C | 108 ± 7 | 104 ± 7 | 111 ± 6 | 106 ± 5 | 107 ± 6 | 107 ± 5 | 116 ± 5 |
| D1 | 108 ± 6 | 103 ± 6 | 109 ± 6 | 103 ± 6 | 106 ± 5 | 105 ± 5 | 112 ± 5 |
| D2 | 108 ± 6 | 102 ± 6 | 103 ± 5 | 104 ± 5 | 105 ± 5 | 104 ± 5 | 108 ± 5 |
| D3 | 109 ± 5 | 91 ± 4 | 93 ± 5 | 95 ± 5 | 98 ± 4 | 96 ± 6 | 98 ± 5 |
| MAP (mmHg) | |||||||
| C | 65 ± 11 | 66 ± 15 | 71 ± 11 | 70 ± 16 | 65 ± 15 | 68 ± 12 | 66 ± 12 |
| D1 | 66 ± 13 | 64 ± 11 | 72 ± 11 | 66 ± 10 | 62 ± 13 | 65 ± 15 | 67 ± 11 |
| D2 | 63 ± 12 | 62 ± 12 | 64 ± 10 | 62 ± 14 | 60 ± 13 | 64 ± 13 | 65 ± 13 |
| D3 | 66 ± 11 | 72 ± 13a | 55 ± 12a | 58 ± 11 | 57 ± 12 | 58 ± 13 | 62 ± 11 |
Results are presented as the mean ± standard deviation.
HR: heart rate; MAP: mean arterial pressure; T1: prior to dexmedetomidine/saline infusion; T2: surgery initiation; T3: at full insufflation; T4: end of surgery; T5: after laryngeal mask removal; T6: after transferring into the postanesthesia care unit (PACU); T7: at PACU discharge.
P < 0.05 vs. group C (analysis of variance with the post hoc Bonferroni test).
Adverse events
One child in group D1 suffered from severe laryngospasm caused by premature drug withdrawal. Symptoms were relieved after application of a pressurized oxygen mask under a deeper anesthesia state induced by intravenous propofol. No complications (e.g., nausea, vomiting, airway obstruction, respiratory depression) were observed in the remaining children. No abnormalities were found during the 24-h postoperative follow-up.
Discussion
The results of the present study suggest that Dex could lower the frequency of EA after general anesthesia in children. It could also improve the postoperative pain score and reduce sevoflurane consumption during general anesthesia.
EA and ED are dissociative states of consciousness in which the child is inconsolable, irritable, uncompromising, and/or uncooperative, typically presenting as thrashing, crying, moaning, and/or being incoherent.1 Despite being a well-known aftereffect of general anesthesia, the pathogenesis of EA and ED is still unclear. Studies suggest that these entities result from the synergistic effect of multiple factors, such as the type of surgery, choice of anesthetic drugs, pain, age and personality of the child, anesthesia duration, the environment, and complementary medicines.17 Voepel–Lewis et al.18 showed that most EA events in children occurred 3–45 min after extubation. As the present study was not designed to study the causative factors of EA and ED, additional studies are necessary to determine the exact mechanisms leading to EA and ED.
Some previous studies suggested that Dex is safe in children.3–5,10,19–22 Some showed that agitation and pain scores were significantly reduced by Dex, although the waking and extubation times were both significantly longer in children administered a single dose of Dex (0.5 µg/kg) 5 min after tonsillectomy23 or in children given Dex 0.5 µg/kg/h intravenously after anesthesia induction.24 Another randomized, controlled trial showed that a continuous infusion of Dex (0.2 µg/kg/h) after anesthesia induction could significantly reduce the frequency of ED by 10%–26%.9 Another study showed that Dex decreased the symptoms of EA after waking, except during the first 30 min,14 a finding that warrants further study. A previous meta-analysis showed that Dex reduced the risk of EA to 0.22 (95% confidence interval 0.14–0.33).1 Among the reviewed studies, only one reported a borderline marginal effect of Dex.25 All of the other studies reported that Dex had a marked effect.1 The present study suggests that infusion of Dex ≥ 0.5 µg/kg within 10 min after anesthesia induction but before surgery significantly decreases the frequency of EA and ED during the first 2 h postoperatively, which is supported by the previous studies cited earlier.
The effects of Dex on the occurrence of EA and ED may be due to the α2AR agonist effect of Dex in the locus ceruleus of the brain stem, producing sedative, hypnotic, and anxiolytic effects via enhanced activation of the inhibitory neurons. Dex also activates α2AR in the spinal dorsal horn and reduces the release of substance P, producing an analgesic effect.26 A study in healthy volunteers27 showed that Dex is a moderate analgesic drug, with its effect reaching a plateau at a dose of approximately 0.5 µg/kg, which may explain why there was no significant difference in ED frequency or CHIPPS scores between groups D2 and D3 in the present study. The surgery-induced stress response could be significantly inhibited by the intrinsic anti-sympathomimetic and analgesic effects of Dex,26 which would reduce the intraoperative consumption of sevoflurane—also an important factor in reducing the frequency of postoperative EA. The hemodynamics were stable, and the transient increase of MAP in group D3 may have resulted from contractions of vascular smooth muscles caused by stimulation of the peripheral vascular α-receptors (α1 and α2β) induced by the rapid infusion of high doses of Dex.28 Studies on whether Dex influences the waking time, however, are controversial,29,30 which may be associated with the different routes of administration, dosages, and withdrawal times used in these studies. In the present study, infusion of Dex at a dose ≥ 0.5 µg/kg could significantly increase TE and TA, and infusion of Dex at a dose of 1 µg/kg could significantly increase TP. Additional studies are needed to determine the best balance among the dose, route of administration, and withdrawal time of Dex.
The drugs used in combination with Dex may also influence the observed effects. Indeed, a meta-analysis showed that the use of propofol, ketamine, fentanyl, and preoperative analgesia had prophylactic effects against EA following sevoflurane anesthesia in children.2 In the present study, propofol, fentanyl, and acetaminophen were used in combination with Dex, and we cannot completely exclude the possibility that these drugs affected the occurrence of EA and ED. Nevertheless, the drugs were used comparably in this study, so it is probable that the observed effects were mainly due to Dex.
Our study has some limitations. First, the sample size was relatively small, and multi-center studies of a pediatric population are needed. Second, infusion concentrations of Dex differed among the groups because drug dilution with equal volumes was required to blind the observers. Third, we did not use any objective indicator to monitor the depths of anesthesia. Although Dex could affect the hemodynamics, we still made adjustments to maintain concentrations based on hemodynamic changes. Finally, evaluation scales for ED were monotonous and subjective, which could lead to errors and require improvement.
Conclusions
Preoperative application of Dex significantly lowered the postoperative frequency of EA and ED, improved the postoperative pain score, and reduced sevoflurane consumption during general anesthesia. A Dex dose of 0.5 µg/kg appears to have better effects than the dose at 0.25 µg/kg. The 1.0 µg/kg dose did not seem to have better efficacy than the 0.5 µg/kg dose. Nevertheless, Dex appears to be safe and efficacious and to have little effect on hemodynamics.
Acknowledgements
The Authors acknowledge the invaluable participation of the patients and their parents.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Pickard A, Davies P, Birnie K, et al. Systematic review and meta-analysis of the effect of intraoperative alpha(2)-adrenergic agonists on postoperative behaviour in children. Br J Anaesth 2014; 112: 982–990. [DOI] [PubMed] [Google Scholar]
- 2.Dahmani S, Stany I, Brasher C, et al. Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: a meta-analysis of published studies. Br J Anaesth 2010; 104: 216–223. [DOI] [PubMed] [Google Scholar]
- 3.Akin A, Bayram A, Esmaoglu A, et al. Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Paediatr Anaesth 2012; 22: 871–876. [DOI] [PubMed] [Google Scholar]
- 4.Mountain BW, Smithson L, Cramolini M, et al. Dexmedetomidine as a pediatric anesthetic premedication to reduce anxiety and to deter emergence delirium. AANA J 2011; 79: 219–224. [PubMed] [Google Scholar]
- 5.Mukherjee A, Das A, Basunia SR, et al. Emergence agitation prevention in paediatric ambulatory surgery: A comparison between intranasal Dexmedetomidine and Clonidine. J Res Pharm Pract 2015; 4: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology 2000; 93: 1345–1349. [DOI] [PubMed] [Google Scholar]
- 7.Piao G, Wu J. Systematic assessment of dexmedetomidine as an anesthetic agent: a meta-analysis of randomized controlled trials. Arch Med Sci 2014; 10: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics 2009; 50: 206–217. [DOI] [PubMed] [Google Scholar]
- 9.Shukry M, Clyde MC, Kalarickal PL, et al. Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Paediatr Anaesth 2005; 15: 1098–1104. [DOI] [PubMed] [Google Scholar]
- 10.Meng QT, Xia ZY, Luo T, et al. Dexmedetomidine reduces emergence agitation after tonsillectomy in children by sevoflurane anesthesia: a case-control study. Int J Pediatr Otorhinolaryngol 2012; 76: 1036–1041. [DOI] [PubMed] [Google Scholar]
- 11.Cole JW, Murray DJ, McAllister JD, et al. Emergence behaviour in children: defining the incidence of excitement and agitation following anaesthesia. Paediatr Anaesth 2002; 12: 442–447. [DOI] [PubMed] [Google Scholar]
- 12.Berthoud MC, Reilly CS. Adverse effects of general anaesthetics. Drug Saf 1992; 7: 434–459. [DOI] [PubMed] [Google Scholar]
- 13.Buttner W, Finke W. Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on seven consecutive studies. Paediatr Anaesth 2000; 10: 303–318. [DOI] [PubMed] [Google Scholar]
- 14.Isik B, Arslan M, Tunga AD, et al. Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatr Anaesth 2006; 16: 748–753. [DOI] [PubMed] [Google Scholar]
- 15.Steward DJ. A simplified scoring system for the post-operative recovery room. Can Anaesth Soc J 1975; 22: 111–113. [DOI] [PubMed] [Google Scholar]
- 16.Ead H. From Aldrete to PADSS: Reviewing discharge criteria after ambulatory surgery. J Perianesth Nurs 2006; 21: 259–267. [DOI] [PubMed] [Google Scholar]
- 17.Mizuno J, Nakata Y, Morita S, et al. Predisposing factors and prevention of emergence agitation. Masui 2011; 60: 425–435. [PubMed] [Google Scholar]
- 18.Voepel-Lewis T, Malviya S, Tait AR. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg 2003; 96: 1625–1630. [DOI] [PubMed] [Google Scholar]
- 19.Chen JY, Jia JE, Liu TJ, et al. Comparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in children. Can J Anaesth 2013; 60: 385–392. [DOI] [PubMed] [Google Scholar]
- 20.Ali MA, Abdellatif AA. Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: A comparison of dexmedetomidine and propofol. Saudi J Anaesth 2013; 7: 296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smania MC, Piva JP, Garcia PC. Dexmedetomidine in anesthesia of children submitted to videolaparoscopic appendectomy: a double-blind, randomized and placebo-controlled study. Rev Assoc Med Bras (1992) 2008; 54: 308–313. [DOI] [PubMed] [Google Scholar]
- 22.Fagin A, Palmieri T, Greenhalgh D, et al. A comparison of dexmedetomidine and midazolam for sedation in severe pediatric burn injury. J Burn Care Res 2012; 33: 759–763. [DOI] [PubMed] [Google Scholar]
- 23.Guler G, Akin A, Tosun Z, et al. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth 2005; 15: 762–766. [DOI] [PubMed] [Google Scholar]
- 24.Simsek M, Bulut MO, Ozel D, et al. Comparison of sedation method in pediatrics cardiac catheterization. Eur Rev Med Pharmacol Sci 2016; 20: 1490–1494. [PubMed] [Google Scholar]
- 25.Asaad OM, Hafez M, Mohamed MY, et al. Comparative study between prophylactic single dose of fentanyl and dexmedetomidine in the management of agitation after sevoflurane anesthesia without surgery. Egypt J Anaesth 2011; 27: 31–37. [Google Scholar]
- 26.Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol 2011; 28: 3–6. [DOI] [PubMed] [Google Scholar]
- 27.Jaakola ML, Salonen M, Lehtinen R, et al. The analgesic action of dexmedetomidine–a novel alpha 2-adrenoceptor agonist–in healthy volunteers. Pain 1991; 46: 281–285. [DOI] [PubMed] [Google Scholar]
- 28.Riker RR, Fraser GL. Adverse events associated with sedatives, analgesics, and other drugs that provide patient comfort in the intensive care unit. Pharmacotherapy 2005; 25: 8 S–18 S. [DOI] [PubMed] [Google Scholar]
- 29.Kim HS, Byon HJ, Kim JE, et al. Appropriate dose of dexmedetomidine for the prevention of emergence agitation after desflurane anesthesia for tonsillectomy or adenoidectomy in children: up and down sequential allocation. BMC Anesthesiol 2015; 15: 79–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pestieau SR, Quezado ZM, Johnson YJ, et al. The effect of dexmedetomidine during myringotomy and pressure-equalizing tube placement in children. Paediatr Anaesth 2011; 21: 1128–1135. [DOI] [PubMed] [Google Scholar]