Abstract
Objectives
This retrospective study used various indices to evaluate remission and low disease activity in ‘real life’ patients with rheumatoid arthritis (RA), given antitumour necrosis factor (anti-TNF) as a first-line treatment; changes in concomitant steroid and conventional synthetic disease-modifying antirheumatic drug (csDMARD) treatment were also assessed.
Methods
Remission and low disease activity were analysed in patients with RA treated with anti-TNF using the 28-joint Disease Activity Score (DAS28), Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI). Remission and low disease activity were recorded after 6 months, 1 year and 2 years, along with concomitant prednisone and csDMARD treatment.
Results
A total of 271 patients with RA were included in the study. After 6 months, remission rates were 18.0%, 20.3% and 23.0% as assessed by CDAI, SDAI and DAS28, respectively. After 1 year and 2 years, respectively, remission rates were 18.4% and 15.9% using CDAI, 21.8% and 17.3% using SDAI, and 22.1% and 17.3% using DAS28. Low disease activity was achieved in 30–40% of patients, depending on the indices used. There was a significant reduction in the number of patients on prednisone and csDMARDs during anti-TNF treatment.
Conclusion
Remission with first-line anti-TNF treatment is an achievable goal in clinical practice, allowing a reduction in concomitant csDMARD and prednisone treatment.
Keywords: Antitumour necrosis factor, disease-modifying antirheumatic drugs, low disease activity, remission, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by systemic and local inflammation that can be partially reversed by treatment.1–3 Biological disease-modifying antirheumatic drugs (DMARDs) render remission or low disease activity as achievable goals.4 Different remission criteria have been developed.5–7 The aim of the present study was to evaluate remission, sustained remission and low disease activity in ‘real life’ patients with RA, treated with first-line antitumour necrosis factor (TNF) drugs using different remission and low disease activity criteria. Changes in concomitant steroid and conventional synthetic DMARD (csDMARD) treatment were also monitored.
Patients and methods
Remission and low disease activity were retrospectively analysed in patients with RA treated with anti-TNF drugs (etanercept 50 mg weekly subcutaneously or adalimumab 40 mg every other week subcutaneously) between January 2008 and December 2014, at the Rheumatology Unit of the University of Rome Tor Vergata, Rome, Italy, using the 28-joint Disease Activity Score (DAS28) (low disease activity <3.2, remission <2.6),5 Simplified Disease Activity Index (SDAI) (low disease activity ≤11, remission ≤3.3)6 and Clinical Disease Activity Index (CDAI) (low disease activity ≤10, remission ≤2.8).7 Patients treated previously with biological agents and those with incomplete follow-up data were excluded from the study.
Remission and low disease activity were recorded 6 months, 1 year and 2 years after the start of anti-TNF treatment, along with concomitant prednisone and csDMARD treatment.
Statistical analyses
Results were reported as the n (%) of patients or mean ± SD. Statistical analyses were performed using McNemar, Fisher or χ2-tests as appropriate. A P-value < 0.05 was considered to be statistically significant. All statistical analyses were performed using GraphPad Prism® version 6 (GraphPad Software, La Jolla, CA, USA).
Results
A total of 271 patients with RA were included in the study, of whom 159 were treated with etanercept and 112 were treated with adalimumab. Demographic and clinical data for the patients are given in Table 1.
Table 1.
Demographic and clinical data for 271 patients with rheumatoid arthritis included in a real-life study investigating first-line antitumour necrosis factor therapy.
| Characteristic | N |
|---|---|
| Sex | |
| Female | 219 (80.8) |
| Male | 52 (19.2) |
| Age, years | 54.5 ± 13.2 |
| Disease duration, years | 8.8 ± 11.1 |
| Early arthritis (<2 years) | 66 (24.3) |
| Positive rheumatoid factor | 173 (63.8) |
| Anti-TNF therapy in combination with csDMARDs | 193 (71.2) |
| Anti-TNF monotherapy | 78 (28.8) |
| Etanercept treatment | 159 (58.7) |
| Adalimumab treatment | 112 (41.3) |
| Prednisone treatment | 137 (50.5) |
| Baseline DAS28 score | 5.2 ± 1.3 |
| Baseline CDAI score | 27.4 ± 14.2 |
| Baseline SDAI score | 28.3 ± 14.7 |
Data presented as n (%) of patients or mean ± SD.
Anti-TNF, antitumour necrosis factor; CDAI, Clinical Disease Activity Index; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; DAS28, 28-joint Disease Activity Score; SDAI, Simplified Disease Activity Index.
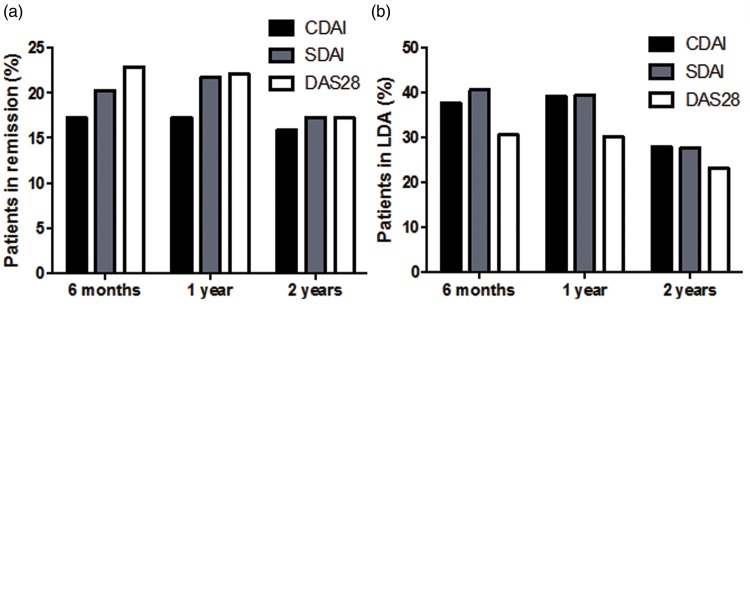
The majority of patients had longstanding disease and at baseline demonstrated high disease activity as assessed by CDAI, SDAI and DAS28 (Table 1). Remission was achieved in 49 (18.0%), 55 (20.3%) and 62 (23.0%) patients after 6 months’ treatment with anti-TNF, in 50 (18.4%), 59 (21.8%) and 60 (22.1%) patients after 1 year’s treatment, and in 43 (15.9%), 47 (17.3%) and 47 (17.3%) patients after 2 years’ treatment, as assessed by CDAI, SDAI and DAS28, respectively (Figure 1a). Remission at two consecutive timepoints was detected in 34 (12.5%) patients as assessed using CDAI and 42 (15.5%) patients as assessed using SDAI and DAS28, while sustained long-term remission (at three timepoints) was observed in 13 (4.8%), 14 (5.2%) and 12 (4.4%) patients as assessed by CDAI, SDAI and DAS28, respectively.
Figure 1.
(a) Proportion of patients in remission assessed using various indices at 6 months, 1 year and 2 years after the start of treatment with antitumour necrosis factor (anti-TNF). (b) Proportion of patients with low disease activity (LDA) assessed using various indices at 6 months, 1 year and 2 years after the start of treatment with anti-TNF. (c) Number of patients treated with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and prednisone (PDN) before and during 2 years’ treatment with anti-TNF. CDAI, Clinical Disease Activity Index; SDAI, Simplified Disease Activity Index; DAS28, 28-joint Disease Activity Score.
Low disease activity was detected in 102 (37.6%), 110 (40.6%) and 83 (30.6%) patients after 6 months’ treatment with anti-TNF, in 107 (39.5%), 107 (39.5%) and 82 (30.2%) patients after 1 year’s treatment, and in 76 (28.0%), 76 (28.0%) and 63 (23.2%) patients after 2 years’ treatment, as assessed by CDAI, SDAI and DAS28, respectively (Figure 1b).
During anti-TNF treatment, there was a significant reduction in the number of patients on prednisone and csDMARDs after 6 months, 1 year and 2 years compared with baseline (P < 0.0001 for all; Figure 1c).
Over the 2-year period, discontinuation of anti-TNF therapy was reported in 51 (18.8%) patients. Reasons for discontinuation were inefficacy in 31 patients (60.8%) and adverse events in 20 patients (39.2%).
Discussion
In the present study, ∼20% of patients with RA treated with first-line anti-TNF drugs in routine clinical care achieved remission and 30–40% achieved low disease activity, depending on the indices used. These data are in agreement with those from the literature.4 The different indices used had different stringencies, with CDAI identifying a smaller proportion of patients in remission than DAS28 and SDAI, although CDAI relies on clinical physical examination and patient global assessment and does not include laboratory parameters. However, only a small proportion of patients (∼5%) achieved sustained long-term remission.
In conclusion, our study findings demonstrate that in real-life clinical practice, remission is an achievable goal using with first-line anti-TNF treatment, allowing a reduction in concomitant csDMARD and prednisone treatment, as suggested by European League Against Rheumatism recommendations.8
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
Editorial assistance was provided by Gayle Robins on behalf of HPS–Health Publishing and Services Srl and funded by Pfizer Italia.
References
- 1.Scrivo R, Conigliaro P, Riccieri V, et al. Distribution of interleukin-10 family cytokines in serum and synovial fluid of patients with inflammatory arthritis reveals different contribution to systemic and joint inflammation. Clin Exp Immunol 2015; 179: 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conigliaro P, Perricone C, Benson RA, et al. Type I IFN system in rheumatoid arthritis. Autoimmunity 2010; 43: 220–225. [DOI] [PubMed] [Google Scholar]
- 3.Conigliaro P, Triggianese P, Perricone C, et al. Restoration of peripheral blood natural killer and B cell levels in patients affected by rheumatoid and psoriatic arthritis during etanercept treatment. Clin Exp Immunol 2014; 177: 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mierau M, Schoels M, Gonda G, et al. Assessing remission in clinical practice. Rheumatology (Oxford) 2007; 46: 975–979. [DOI] [PubMed] [Google Scholar]
- 5.Prevoo ML, van’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995; 38: 44–48. [DOI] [PubMed] [Google Scholar]
- 6.Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003; 42: 244–257. [DOI] [PubMed] [Google Scholar]
- 7.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005; 23(suppl 39): 100–108. [PubMed] [Google Scholar]
- 8.Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014; 73: 492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]