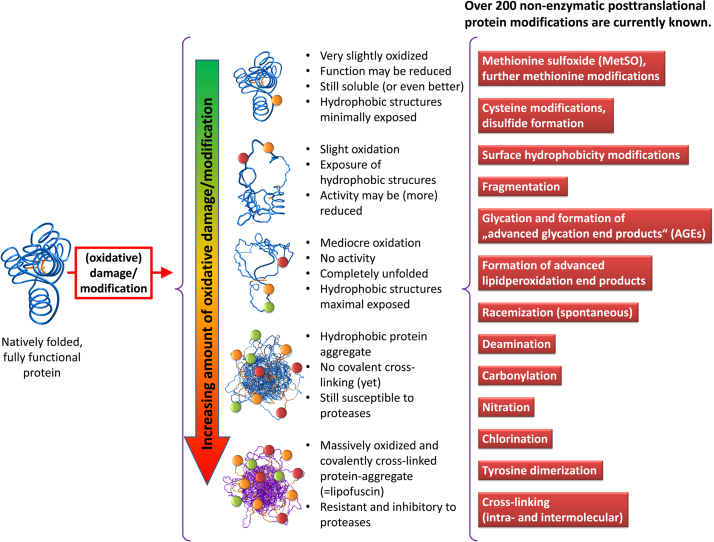
Fig. 1.
Oxidation of a soluble protein. The degree of oxidative damage applied to a native protein is both time- and dose-dependent. Minimal amounts of damage may show only slight or no impact on protein function, solubility in this case may even increase, since additional charges are introduced into the protein. Further oxidation leads to a partial unfolding and exposure of hydrophobic residues that are normally buried inside soluble proteins, the overall solubility now decreases compared to the native form of the protein. Mediocre oxidation results in further/complete loss of activity and entire unfolding, hydrophobic structures are now fully exposed. Larger protein aggregates are formed by hydrophobic interactions of such unfolded proteins; formation of such aggregates is still reversible, since the single proteins are not covalently cross-linked. Further oxidation leads to a largely covalently cross-linked protein-aggregate; formation of those structures is irreversible, these products are highly resistant to mammalian proteases. The list on the right shows the most important of the over 200 currently known enzymatic and non-enzymatic posttranslational protein modifications.