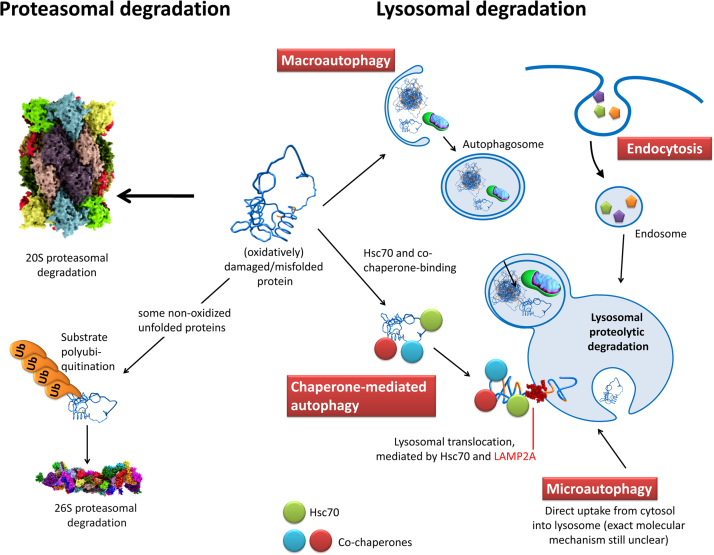
Fig. 7.
The possible degradation-pathways of an (oxidatively) damaged/unfolded protein. The UPS-pathway - a damaged protein can be degraded by the 20S proteasome or the 26S proteasome. However, polyubiquitination is not a pathway for oxidatively damaged proteins, since those are not preferentially polyubiquitinated [198], [199]. Macroautophagy - a phagophore engulfs a cytosolic volume, that may also contain single damaged proteins, but mainly larger protein-aggregates (can be covalently cross-liked or not) or even whole organelles such as aged/dysfunctional mitochondria. The resulting autophagosome containing the lysosomal substrates fuses with a lysosome, exposing those substrates to a large variety of enzymes. Chaperone-mediated autophagy - a substrate protein is bound by different chaperones as Hsc70 and its co-chaperones and is translocated into the lysosomal system via interaction of Hsc70 and the “lysosome-associated membrane protein 2” (LAMP-2A). LAMP-2A translocates the substrate into the lysosomal volume where it is bound by a lysosomal form of Hsc70 (lys-Hsc70). Microautophagy - substrates are directly taken up from the cytosol via invagination by the lysosomal system. Endocytosis - substrates from the outside of the cell are taken up by the cell via an invagination of the cell membrane, forming an endosome that fuses with the lysosomal system, resulting in substrate degradation.