Abstract
Background
Myasthenia gravis is an autoimmune disease of the neuromuscular junction, commonly affecting the ocular muscles. Cigarette smoking has been shown to influence many autoimmune diseases, including multiple sclerosis and rheumatoid arthritis, but its effect on myasthenia gravis has not been well studied. We sought to determine whether cigarette smoking influenced disease-related symptoms in ocular myasthenia gravis (OMG).
Methods
We performed a prospective, clinic-based cross-sectional study in a single academic neuro-ophthalmology practice. All patients diagnosed with OMG between November 2006 and April 2014 were included. A prospective telephone survey was administered to determine smoking status and myasthenia gravis-related symptom severity. The main outcome measure was the myasthenia gravis-specific activities of daily living (MG-ADL) score, a well-validated marker of symptoms and quality of life in myasthenia gravis.
Results
Forty-four patients were included in the analysis. Comparison of MG-ADL ocular subscores between current smokers (3.4 ± 2.6), former smokers (1.8 ± 2.1), and never smokers (1.1 ± 1.5) revealed a statistically significant relationship (P = 0.031) where current smokers had the highest MG-ADL ocular subscores and never smokers the lowest. Comparison of MG-ADL total scores revealed the same relationship (current 5.6 ± 4.5, former 2.9 ± 3.1, never 1.4 ± 2.5, P = 0.003). There were borderline significant correlations of pack years with MG-ADL ocular subscore (r = 0.27, P = 0.074) and MG-ADL total score (r = 0.30, P = 0.051).
Conclusions
Our findings indicate an association between cigarette smoking and symptom severity in OMG. This association suggests that smoking cessation in OMG patients may lead to improved symptom-related quality of life.
Myasthenia gravis (MG) is an autoimmune disorder characterized by inflammation at the neuromuscular junction. The condition is most often caused by autoantibodies targeting the postsynaptic skeletal muscle nicotinic acetylcholine receptor (AChR) (1). Impaired neuromuscular transmission leads to fluctuating weakness, which can affect any skeletal muscle. Extraocular muscle involvement is common resulting in diplopia and ptosis. When disease activity is limited to the eye muscles, the disease is termed ocular myasthenia gravis (OMG).
Cigarette smoking and other forms of chronic nicotine use have been shown to alter AChRs, but this research has focused on neuronal receptors in the brain (2). Although cigarette smoking has been shown to cause or exacerbate other autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and Graves disease (3), the effect of cigarette smoking on muscle nAChR and on MG is not clear. Maniaol et al (4) found a higher prevalence of MG among cigarette smokers as compared with the general population, and Moreau et al (5) described a patient who had an exacerbation of MG after transdermal application of nicotine. Aside from these 2 reports, little is known about whether cigarette smoking may play a causal role, influence disease activity, or exacerbate symptoms and quality of life in MG.
We surveyed patients with ocular symptoms from myasthenia gravis to determine whether cigarette smoking influenced symptom severity and quality of life in MG as determined by the myasthenia gravis-specific activities of daily living (MG-ADL) total score and ocular subscore.
METHODS
We undertook a clinic-based cross-sectional study by performing a scripted, interviewer-administered telephone survey to all patients who received the diagnosis of OMG in our academic neuro-ophthalmology practice from November 2006 to April 2014. OMG was defined as weakness limited to the extraocular muscles, eyelid levators, and orbicularis oculi (6) with at least one of the following pieces of supplementary evidence for the disease: 1) serum anti-AChR antibodies, 2) unequivocal improvement of symptoms with intravenous injection of edrophonium, or 3) a typical clinical presentation with symptom improvement after oral corticosteroids and/or acetylcholinesterase inhibitors. Many patients had multiple visits to our clinic, and they were deemed to have OMG if they met these diagnostic criteria during any of those visits. Electromyography was not used as a diagnostic criterion because we do not routinely perform such testing in patients with purely ocular symptoms. Patients were excluded from the study if they were unable to be contacted, did not give consent, or if their diagnosis of MG was uncertain. Patients were also excluded if they had a confounding diagnosis such as thyroid eye disease. Patients who developed symptoms of generalized MG between initial diagnosis and survey administration were not excluded; however, those who had generalized symptoms before diagnosis were excluded. This judgment was made based on clinical history. Survey administration took place between February and April of 2014.
Smoking status was determined with questions modified from the National Health Interview Survey (7). The MG-ADL (activities of daily living) score, a well-validated tool for measuring outcomes in MG (8,9), was administered to determine symptom severity and quality of life. The last 2 questions of the MG-ADL score pertaining to ocular symptoms were analyzed separately as an ocular subscore (See Supplemental Digital Content, Figure E1, http://links.lww.com/WNO/A175). In the MG-ADL score, higher scores indicate greater symptom severity. MG-ADL scores were compared between smoking groups with analysis of variance and also in a general linear model with covariate adjustment for age, gender, and months since disease onset. Approval was obtained before study initiation from the Institutional Review Board at the University of Miami, verbal informed consent was obtained from all participants, the study was HIPAA compliant, and the study adhered to the tenets of the Declaration of Helsinki.
RESULTS
Eighty-four patients with MG were identified during the study period; all presented to our clinic with ocular symptoms. Thirteen were excluded due to an uncertain or potentially confounding diagnosis, and 15 were excluded due to an inability to obtain correct contact information. Phone calls were made to 56 patients, but 11 could not be reached and 1 declined to participate. At least 3 attempts were made to contact all patients. In all, surveys were administered to 44 of 45 patients who were reachable with a response rate of 98%. Survey respondents were divided into 3 groups: current smokers, former smokers, and never smokers. The characteristics of these 3 groups are summarized in the Table 1.
TABLE 1.
Patients with ocular myasthenia gravis and their smoking history
| Current (N = 7) | Former (N = 16) | Never (N = 21) | P | Total (N = 44) | |
|---|---|---|---|---|---|
| N (%) | |||||
| Male gender | 3 (43) | 14 (88) | 13 (62) | 0.083 | 30 (68) |
| Female gender | 4 (57) | 2 (12) | 8 (38) | 14 (32) | |
| Mean (SD) | |||||
| Age, y | 50 (14) | 69 (15) | 48 (25) | 0.010 | 56 (22) |
| Time from onset to survey, y | 5.3 (9.7) | 1.4 (3.1) | 2.3 (5.5) | 0.32 | 2.5 (5.7) |
| Mean ± SD | |||||
| MG-ADL score | 5.6 ± 4.5 | 2.9 ± 3.1 | 1.4 ± 2.5 | 0.003 | |
| MG-ADL ocular subscore | 3.4 ± 2.6 | 1.8 ± 2.1 | 1.1 ± 1.5 | 0.031 | |
ADL, activities of daily living; MG, myasthenia gravis.
Thirty-seven of the 44 patients had a positive edrophonium test or AChR antibodies or both, the remaining 7 patients had a typical clinical presentation with symptoms that improved with oral corticosteroids and/or acetylcholinesterase inhibitors. At the time of the survey, 11 patients were being treated with prednisone alone, 6 with pyridostigmine alone, 10 with both prednisone and pyridostigmine, 6 with steroid sparing agents, and 11 were receiving no treatment. A breakdown of the treatments that each smoking group was receiving at the time of the survey can be viewed online as Supplemental Digital Content, Figure E2, http://links.lww.com/WNO/A176. Ten of the 44 patients had developed generalized symptoms by the time of survey administration. Of these 10, 9 were either former or current smokers.
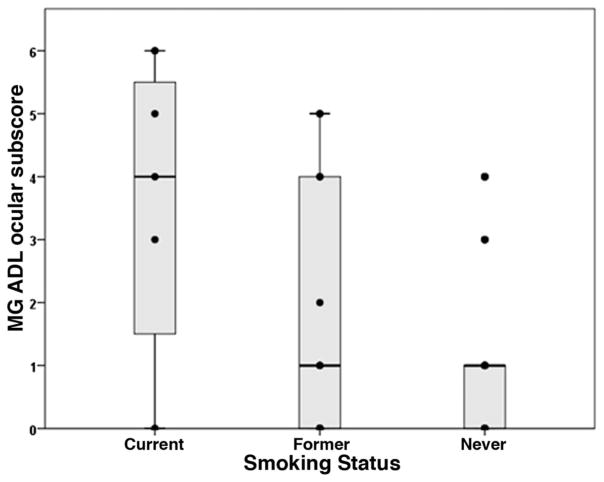
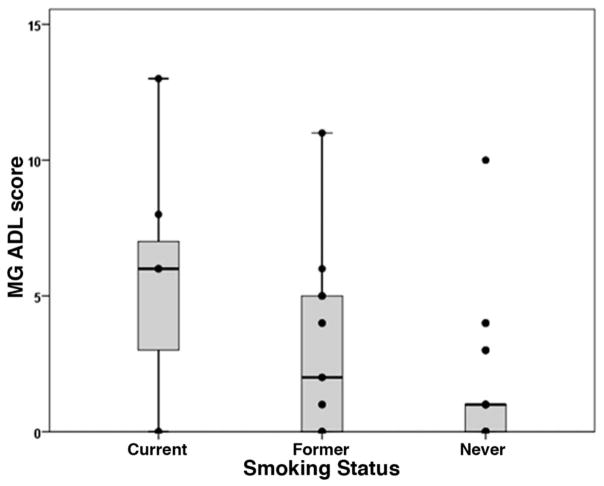
Comparison of MG-ADL ocular subscores between current smokers (3.4 ± 2.6), former smokers (1.8 ± 2.1), and never smokers (1.1 ± 1.5) revealed a statistically significant relationship (P = 0.031, one way analysis of variance) where current smokers had the highest MG-ADL ocular subscores and never smokers the lowest (Table 1 and Fig. 1). Comparison of MG-ADL total scores revealed the same relationship (current 5.6 ± 4.5, former 2.9 ± 3.1, never 1.4 ± 2.5, P = 0.003, Table 1 and Fig. 2). There were borderline significant correlations of pack years with MG-ADL score (r = 0.30, P = 0.051) and MG-ADL ocular subscore (r = 0.27, P = 0.074). There was no significant correlation between years since smoking cessation and MG-ADL ocular subscore (r = −0.17, P = 0.49) or total score (r = −0.17, P = 0.54).
FIG. 1.
Plot of MG-ADL ocular subscore for each smoking group showing that the median (horizontal bar) is greater for current smokers as compared with former and never smokers (P = 0.007). ADL, activities of daily living; MG, myasthenia gravis.
FIG. 2.
Plot of MG-ADL score for each smoking group showing that the median (horizontal bar) is greater for current smokers as compared with former smokers and never smokers (P = 0.003). ADL, activities of daily living; MG, myasthenia gravis.
One way analysis of variance followed by post hoc least significant difference tests demonstrated that former smokers with MG were older than either current or never smokers (P ≤ 0.045). There were also an unexpectedly high (88%) number of men in the former smokers group. Statistically adjusting for differences in age/gender between the smoking groups did not alter the statistical significance of the difference in MG-ADL ocular subscore or total score by smoking groups (P = 0.006 and P = 0.003, respectively). Statistically adjusting for time from disease onset to survey administration (i.e., disease duration) also did not alter the statistical significance of the differences between groups for both the MG-ADL ocular subscore and total score (P = 0.0031 and P = 0.0034, respectively).
DISCUSSION
Our study demonstrated a significant association between cigarette smoking and symptom severity in patients with OMG. Current smokers had the highest MG-ADL ocular subscores and the highest MG-ADL total scores while never smokers had the lowest scores. Former smokers had scores between these 2 groups. The suggestion of a relationship between pack years and MG-ADL scores was also discovered, but this did not reach statistical significance. In light of the results of this study, we urge clinicians to strongly recommend smoking cessation to myasthenic patients in the hopes that successful smoking cessation may lead to improved MG symptoms. Although this study does not specifically address whether OMG symptoms and quality of life may improve after smoking cessation, the overwhelming health benefits from smoking cessation support this recommendation. Furthermore, clinicians may consider providing specific assistance for smoking cessation (e.g., self-help guidance, counseling, medications, and referral to state smoking quitlines) because patients have greater success in quitting as measured by 12-month abstinence rates with assistance as compared with without assistance (10).
Either an alteration of the immune system or of the neuromuscular junction or a combination of both may trigger an effect of cigarette smoking on the MG-ADL score. Cigarette smoking is known to have myriad effects on the immune system. It increases the recruitment of neutrophils, macrophages, and monocytes, and causes increased release of tissue-damaging matrix metalloproteinases as well as other inflammatory cytokines (3). By these and other immune-mediated mechanisms, several autoimmune diseases have been causally linked to smoking, including rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and Graves disease (3).
Alternatively, an effect of cigarette smoking on the physiology of the neuromuscular junction could underlie this association. Prolonged exposure to nicotine results in desensitization or inactivation of neuronal AChR (2). This effect has been shown only for neuronal AChR, and the effect of such exposure on muscle AChRs is unknown. The association discovered here raises the question of whether AChR changes at the neuromuscular junction might be a plausible mechanism for greater symptoms in myasthenics who smoke, especially given that myasthenic neuromuscular junctions are already altered and devoid of AChRs (11,12). Ultimately, this study was not constructed to address mechanism; and these proposed mechanisms remain speculative.
Potential limitations of our study include the possibility of confounding variables that result from its observational nature. Although we were able to statistically adjust for certain variables (age, gender, and disease duration), the possibility of confounding variables persists. In particular, differences in medication usage between smoking groups could lead to differences in the groups’ MG-ADL scores. Smokers are less compliant regarding the treatment for hypertension (13), hyperlipidemia (14), chronic obstructive pulmonary disease (15), and other diseases (16); therefore, it is possible that the current and former smokers in this study were less compliant with MG therapy, which could contribute to a difference in the MG-ADL scores. There is also the possibility of selection bias and recall bias in particular pertaining to survey respondent’s smoking history. Additional prospective, longitudinal studies are warranted to further elucidate the effect of cigarette smoking on the clinical course of patients with OMG.
Although all of the patients included had ocular symptoms of myasthenia gravis, some (10 of 44) went on to develop generalized symptoms. Nine of 10 patients who developed generalized symptoms were either former or current smokers. These patients were not excluded from analysis. Although excluding these patients would have maintained a patient population that was more purely ocular, this may have compromised the clinical application of these data because development of generalized symptoms is a part of the natural history of the disease and can be expected in approximately half of ocular myasthenics (6,17). Further research is necessary to establish whether cigarette smoking may make a patient with OMG more likely to develop generalized MG.
We chose to include edrophonium testing and a response to MG medications as supportive evidence of the diagnosis to include seronegative OMG patients. While specific, the sensitivity of anti-AChR antibodies for OMG is estimated to be between 0.39 and 0.71 (18). Relying on the presence of these antibodies solely to make the diagnosis of OMG would exclude a number of patients who have the disease.
Supplementary Material
Acknowledgments
Supported by NIH Center Core Grant P30EY014801 and a Research to Prevent Blindness Unrestricted Grant.
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the full text and PDF versions of this article on the journal’s Web site (www.jneuro-ophthalmology.com).
STATEMENT OF AUTHORSHIP
Category 1: a. Conception and design: S. M. Gratton and B. L. Lam; b. Acquisition of data: S. M. Gratton and A. M. Herro; c. Analysis and interpretation of data: S. M. Gratton, W. J. Feuer, and B. L. Lam. Category 2: a. Drafting the article: S. M. Gratton; b. Revising it for intellectual content: S. M. Gratton, A. M. Herro, W. J. Feuer, and B. L. Lam. Category 3: a. Final approval of the completed article: S. M. Gratton, A. M. Herro, W. J. Feuer, and B. L. Lam.
References
- 1.Sieb JP. Myasthenia gravis: an update for the clinician. Clin Exp Immunol. 2013;175:408–418. doi: 10.1111/cei.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindstrom J. Nicotinic acetylcholine receptors in health and disease. Mol Neurobiol. 1997;15:193–222. doi: 10.1007/BF02740634. [DOI] [PubMed] [Google Scholar]
- 3.Constenbader K, Karlson E. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15:737–745. doi: 10.1177/0961203306069344. [DOI] [PubMed] [Google Scholar]
- 4.Maniaol A, Boldingh M, Brunborg C, Harbo HF, Tallaksen CME. Smoking and socioeconomic status may affect myasthenia gravis. Eur J Neurol. 2013;20:453–460. doi: 10.1111/j.1468-1331.2012.03843.x. [DOI] [PubMed] [Google Scholar]
- 5.Moreau T, Vandenbeele S, Depierre P, Confavreaux C. Nicotine-sensitive myasthenia gravis. Lancet. 1994;344:548–549. doi: 10.1016/s0140-6736(94)91944-5. [DOI] [PubMed] [Google Scholar]
- 6.Benatar M, Sanders DB, Wolfe GI, McDermott MP, Tawil R. Design of the Efficacy of Prednisone in the Treatment of Ocular Myasthenia (EPITOME) trial. Ann N Y Acad Sci. 2012;1275:17–22. doi: 10.1111/j.1749-6632.2012.06780.x. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed September 20, 2013];National Health Interview Survey Adult Tobacco Use—Guide for Data Users. 2014 Available at: www.cdc.gov/nchs/nhis/tobacco/tobacco_guide.htm#2004_to_present.
- 8.Wolfe GI, Herbelin R, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology. 1999;52:1487. doi: 10.1212/wnl.52.7.1487. [DOI] [PubMed] [Google Scholar]
- 9.Muppidi S, Wolfe GI, Conaway M, Burns T the MG Composite and MG-QOL 15 Study Group. MG-ADL: still a relevant outcome measure. Muscle Nerve. 2011;44:727–731. doi: 10.1002/mus.22140. [DOI] [PubMed] [Google Scholar]
- 10.Zhu S, Melcer T, Sun J, Rosbrook B, Pierce JP. Smoking cessation with and without assistance. Am J Prev Med. 2000;18:305–311. doi: 10.1016/s0749-3797(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 11.Fambrough DM, Drachman DB, Satyamurti S. Neuromuscular junction in myasthenia gravis: decreased acetylcholine receptors. Science. 1973;182:293–295. doi: 10.1126/science.182.4109.293. [DOI] [PubMed] [Google Scholar]
- 12.Serra A, Ruff R, Leigh RJ. Neuromuscular transmission failure in myasthenia gravis: decrement of safety factor and susceptibility of extraocular muscles. Ann N Y Acad Sci. 2012;1275:129–135. doi: 10.1111/j.1749-6632.2012.06841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyngas H, Lahdenera T. Compliance of patients with hypertension and associated factors. J Adv Nurs. 1999;29:832–839. doi: 10.1046/j.1365-2648.1999.00962.x. [DOI] [PubMed] [Google Scholar]
- 14.Kiortsis DN, Giral P, Bruckert E, Turpin G. Factors associated with low compliance with lipid-lowering drugs in hyperlipidemic patients. J Clin Pharm Ther. 2000;25:445–451. doi: 10.1046/j.1365-2710.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 15.Turner J, Wright E, Mendella L, Anthonisen N. Predictors for patient adherence to long-term home nebulizer therapy for COPD. The IPPB Study Group. Chest. 1995;108:394–400. doi: 10.1378/chest.108.2.394. [DOI] [PubMed] [Google Scholar]
- 16.Jin J, Sklar GE, Oh VMS, Li SC. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther Clin Risk Manag. 2008;4:269–286. doi: 10.2147/tcrm.s1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson NP, Deans J, Compston DAS. Myasthenia gravis: a population based epidemiological study in Cambridgeshire. J Neurol Neurosurg Psychiatry. 1998;65:492–496. doi: 10.1136/jnnp.65.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006;16:459–467. doi: 10.1016/j.nmd.2006.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.