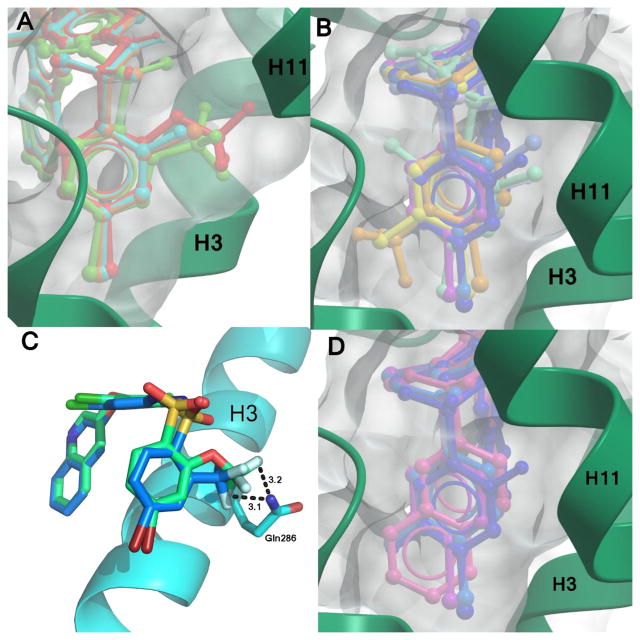
Figure 6.
Effective space filling within the PPARγ LBD binding pocket has been shown to correlate with higher affinities. Displayed are superimpositions of the compound 1 analogs (colored sticks) bound to PPARγ (ribbons, green or blue), and a surface representation of the ligand binding pocket (gray surface). Ligands with substitutions at (A) position 2 of ring A have better packing within the pocket and higher affinities than (B) those which have substitutions at other positions. Ligands with substitutions at position 2 include 3 (red), 4 (orange), 6 (green), and 8 (cyan). Ligands with additional substitutions at positions 3 or 5 have lower affinities and include 2 (yellow), 5 (pale orange), 9 (teal), 11 (purple), 2-chloro-N-(3,5-dichloro-4-(quinolin-3-yloxy)phenyl)benzenesulfonamide (13) (light blue), 14 (turquoise), 15 (lilac), and 10 (dark blue). (C) The oxygen linker in the trifluoro substitution of 3 enables it to reach further into the binding pocket than 6 and confer a better lock-and-key fit in addition to forming additional hydrogen bonds with Gln286. (D) The naphthalene moiety of 12 (pink) extends as far into the binding pocket as other ligands, as shown by comparison with 9 (teal), 10 (dark blue), and 11 (purple).