Abstract
Background
Allergic bronchopulmonary aspergillosis (ABPA) in children with asthma, not associated with cystic fibrosis, is yet to receive the recognition it deserves.
Objective
To highlight the presentation of ABPA in children with asthma.
Methods
This retrospective review documents the occurrence of pediatric ABPA over a period of 31 years in one unit. Children with asthma, eosinophilia and infiltrates on chest radiograph were screened for ABPA. In these patients, demonstration of immediate hypersensitivity response against Aspergillus species along with serological profile and pulmonary function testing were done. Bronchography/computed tomography (CT) of the chest demonstrated central bronchiectasis (CB). CT of the paranasal sinuses was done in patients with upper airways symptoms. In those suspected with allergic Aspergillus sinusitis (AAS) consent was sought from the parents for the invasive procedure needed for the diagnosis of AAS.
Results
Of the 349 patients with ABPA diagnosed, 42 (12.03%) were in the pediatric age group. The mean age on presentation was 12.9 ± 4 years with a male preponderance. All patients had asthma and positive intradermal/skin prick test against Aspergillus species. Ring shadows, the most common radiological presentation, were seen in 28 of 42 patients. Bronchography/CT of the chest demonstrated CB, a feature pathognomic of ABPA, in 32 of 42 patients. High attenuation mucus plugs was observed in 7 of 36 patients while ABPA-seropositive was diagnosed in 10 of 42 patients. On imaging, sinusitis was seen in 20 of 30 patients with upper airways symptoms of whom eight had suspected AAS. Three parents consented for surgery, which confirmed the diagnosis.
Conclusion
This study highlights the need to evaluate asthmatic children for ABPA as also to exclude AAS.
Keywords: Asthma; Aspergillosis, allergic bronchopulmonary; Allergic Aspergillus sinusitis; Central bronchiectasis; High attenuation mucus plugs
INTRODUCTION
Allergic aspergillosis of the respiratory tract in children includes Aspergillus induced asthma, allergic bronchopulmonary aspergillosis (ABPA) and allergic Aspergillus sinusitis (AAS) [1]. It is now well established that Aspergillus sensitization in susceptible patients with asthma leads to a more severe form of the disease. This occurs when Aspergillus spores are entrapped in the viscid sputum triggering a cascade of inflammatory reaction resulting in one or the other form of allergic aspergillosis [1]. In 1952, Hinson et al. [2] from United Kingdom (UK) first documented ABPA in three adult patients. The first pediatric patient too was reported from UK in 1959 in a 15 and a half-year-old boy with “Schonlein-Henoch” purpura [3].
In the pediatric population, ABPA has mostly been observed in children with cystic fibrosis (CF) [4,5]. Only a few reports have documented the occurrence of ABPA in children with asthma [6,7,8,9,10,11,12]. It is important to highlight this disease entity in children as delayed diagnosis leads to irreversible lung damage, morbidity and even mortality. Furthermore, the concomitant occurrence of upper airways symptoms and AAS in these children is yet to receive the attention that it deserves. Over a period of 31 years, diagnosis of ABPA was established in 349 patients in one unit. Forty-two of these patients were 18 years or below and were categorized as pediatric ABPA. This also includes seven patients documented earlier for their unusual presentation [13,14,15,16,17,18]. The paucity of data in the literature prompted this review of 42 asthmatic children with ABPA. To our knowledge, this report documents the largest series of ABPA in children with asthma.
MATERIALS AND METHODS
Pediatric patients <18 years of age with a history of asthma, eosinophilia along with infiltrates on chest radiograph or with history of multiple courses of antituberculous drugs despite persistently negative sputum for acid fast bacilli were screened for ABPA. All patients diagnosed with ABPA were also evaluated for sinusitis. The patients/parents were interviewed regarding symptoms, duration of illness and previous history of intake of antituberculous therapy. Family members with atopy and similar symptoms were also investigated for ABPA.
Intradermal tests against Aspergillus species were done until the availability of skin prick tests antigens in 2007. Serum total IgE, specific IgG, IgE, and serum precipitins against Aspergillus species were also documented. All patients underwent either bronchography or computed tomography (CT) of the chest for evaluation of central bronchiectasis (CB). Bronchography was the initial modality used to demonstrate CB until the advent of CT. Pulmonary function testing in form of spirometry was attempted in all patients.
The diagnosis of ABPA was based on Rosenberg-Patterson [19,20] criteria (8 major and 3 minor criteria). The major criteria included (1) history of asthma, (2) fleeting opacities/transient pulmonary infiltrates, (3) CB, (4) bands of precipitins against Aspergillus species, (5) type I immediate hypersensitivity reaction, (6) absolute eosinophil count, (7) positive specific IgG/IgE against Aspergillus fumigatus, and (8) serum total IgE. The 3 minor criteria included (1) history of occasional passage of brownish plugs along with sputum, (2) sputum culture positive for A. fumigatus, and (3) type III (late) hypersensitivity with extracts of Aspergillus species.
In patients with nasal symptoms along with evidence of sinusitis, X-ray/CT of the paranasal sinuses (CT-PNS) was also performed. If AAS was suspected, consent was sought from the parents to obtain mycological and histopathological specimens. Patients for whom consent was obtained underwent Caldwell-Luc operation until functional endoscopic sinus surgery became available. The criteria adopted by us earlier [21,22,23,24] and later enunciated by deShazo and Swain [25] was followed for the diagnosis of AAS. These included (1) sinusitis of one or more PNS on roentgenogram, (2) necrosed amorphous tissue along with edematous polyps infiltrated with eosinophils on histopathological evaluation of material from the sinuses, (3) demonstration of fungal elements in nasal discharge or in material obtained at the time of surgery by stain or culture, (4) absence of diabetes, previous or subsequent immunodeficiency disease and treatment with immunosuppressive drugs, and (5) absence of invasive fungal disease at the time of diagnosis or subsequently. In addition, this was also supported by other diagnostic criteria seen in ABPA [26].
The data was recorded in Microsoft Excel (Redmond, WA, USA) and expressed as mean ± standard deviation. Vallabhbhai Patel Chest Institutional Ethics Committee approved review of the data.
RESULTS
A total of 349 patients were diagnosed with ABPA over a period of 31 years from 1985 to 2016 in one unit. Of these 349 patients, 42 (12.03%) were in the pediatric age group (range, 3.5–18 years; mean age, 12.97 ± 4.02 years). There were 26 boys and 16 girls (male to female ratio, 1.62). The demographic data, laboratory parameters and diagnostic criteria met by our patients have been detailed in Table 1.
Table 1. Demographic characteristics and diagnostic criteria of allergic bronchopulmonary aspergillosis met (n = 42).
Values are presented as mean ± standard deviation, number, or number (%).
CT, computed tomography.
Cough seen in all 42 (100%) and breathlessness in 41 of 42 patients (97.6%) were the two most common presenting symptoms. Upper airways symptoms were documented in 30 of 42 patients (71.4%). Nasal discharge and sneezing were seen in 24 of 30 (57.1%) and 23 of 30 patients (54.7%), respectively. Constitutional symptoms included fever in 14 of 42 (30%) and loss of weight/appetite in 7 of 42 patients (16.6%) each. Clubbing and cyanosis was seen in 2 of 42 patients each one of whom was documented earlier [14]. Thirteen patients (30.9%) had received antituberculous therapy prior to referral. A familial history of asthma was present in 11 of 42 patients (26.2%) and familial ABPA was documented in 3 pairs of first-degree relatives, which also includes a pair reported earlier [17]. Of the 3 pairs with familial ABPA, 2 were parent-child and one of siblings.
The mean serum IgE was 4,509.16 ± 7,907.77 kUA/L (range, 160–45,600 kUA/L). Type I hypersensitivity was positive in all 42 patients, with A. fumigatus positive in 38 of 42 (90.5%), A. flavus in 29 of 42 (69%), A. tamarii in 22 of 42 (52.4%), and A. niger in 15 of 42 (35.7%). Type III hypersensitivity was documented in 22 of 42 patients (52.4%). Specific IgE against Aspergillus species was positive in 29 of 30 patients (96.7%) where it was done and specific IgG against Aspergillus species was positive in 33 of 42 patients (78.6%). Bands of precipitins against Aspergillus species were detected in 26 of 42 patients (61.9%) with A. fumigatus, the most common one, being positive in 15 of 42 (35.7%) followed by A. flavus in 11 of 42 (26.2%), A. niger in 5 of 42 (11.9%) and A. terreus in 1 of 42 subjects (2.4%). In 5 of 42 subjects (11.9%), precipitin bands against more than one Aspergillus species was observed. Sputum cultured A. fumigatus in 2 of 42 patients.
Spirometry was done in 34 of 42 children. Obstructive ventilatory defect, the most common abnormality, was seen in 14 of 34 (41.2%) followed by a mixed defect in 4 of 34 (11.8%). A restrictive pattern was seen in 3 of 34 patients (8.8%). Normal spirometry was documented in 13 of 34 patients (38.2%). Significant bronchodilator reversibility was seen in all patients with an obstructive defect except one.
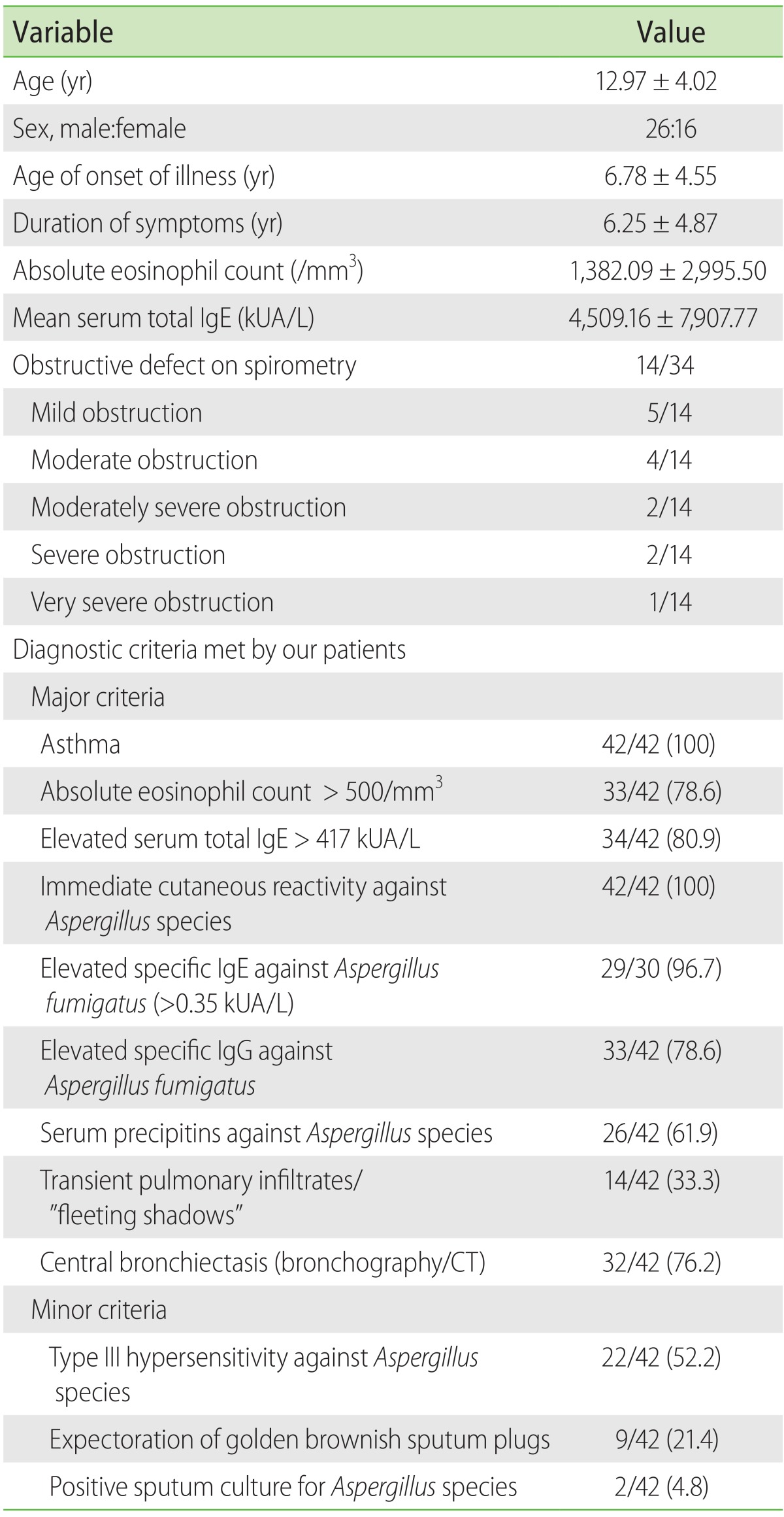
On chest radiography, ring shadows were the most common finding seen in 28 of 42 patients (66.7%) followed by consolidation in 24 of 42 (57.1%) and “fleeting opacities”/transient pulmonary infiltrates (Fig. 1) in 14 of 42 patients (33.3%). In addition, parallel lines were seen in 10 of 42 subjects (23.8%), hilar adenopathy [16] in 7 of 42 (16.7%), v-y shadows in 6 of 42 (14.3%), lobar collapse and gloved finger appearance in 2 of 42 (4.8%) each and cavitation [14] and middle lobe syndrome [18] in 1 of 42 patients (2.4%) each. The right upper zone was involved in 20 of 42 patients (47.6%), left lower zone in 18 of 42 (42.8%), right middle zone in 17 of 42 (40.5%), right lower zone in 15 of 42 (35.7%), left mid zone in 11 of 42 (26.2%), and left upper zone in 8 of 42 patients (19%).
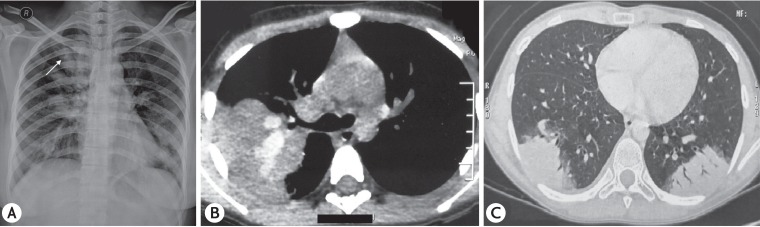
Fig. 1. (A) Chest radiograph of an 11-year-old child showing right upper zone infiltrates. (B) Chest radiograph of the same child 12 months later showing left lower zone infiltrates. (C) Chest radiograph 3 months later than panel B showing right lower zone infiltrates. (D) Chest radiograph showing clearing of the infiltrates after therapy.
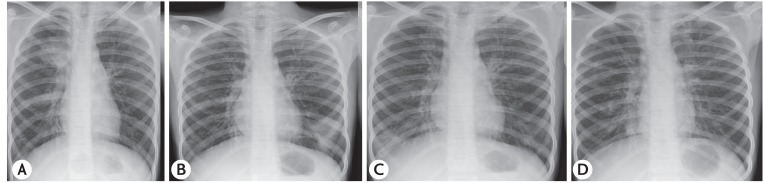
CB with normal peripheral tapering was documented in 32 of 42 patients (76.2%) (4 of 6 on bronchography [Fig. 2A] and 28 of 36 patients on CT chest). Bronchography was done in 6 of 42 patients (14.3%) while CT of the chest was performed in 36 of 42 (85.7%). “Signet ring” appearance (Fig. 2B) was seen in 24 of 36 (66.7%) while “strings of pearl” appearance (Fig. 2B) was reported in 6 of 36 subjects (16.7%). CB on CT of the chest was documented in 28 of 36 patients (77.8%) (cystic bronchiectasis in 18 of 36 [50%], cylindrical bronchiectasis in 11 of 36 [30.6%] and varicose bronchiectasis in 6 of 36 patients [16.7%]). Based on demonstration of CB, 32 of 42 (76.2%) were categorized as ABPA-CB while 10 of 42 (23.8%) were labeled as ABPA-seropositive (ABPA-S). The CT findings have been detailed in Table 2.
Fig. 2. (A) Bronchographic image showing central bronchiectasis with normal peripheral tapering of the right upper lobe bronchus. (B) High resolution computed tomography of the chest (lung window) showing central bronchiectasis with “string of pearls” appearance (white arrow) and “signet ring” (open arrow).
Table 2. Computed tomography findings* in children with allergic bronchopulmonary aspergillosis (n = 36).
*As per frequency.
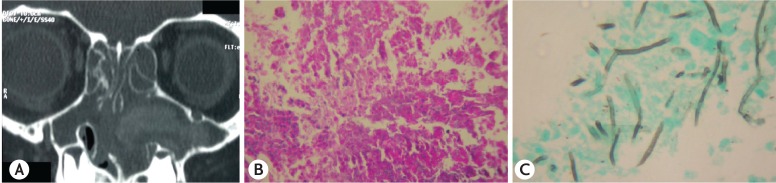
Of the 30 patients with upper airways symptoms, X-ray of the PNS was done in 4 and CT-PNS in 26 patients. Sinusitis was documented in 20 of 30 patients (66.7%) (X-ray PNS, 2 of 4 [50%]; CT-PNS, 18 of 26 [69.2%]). Maxillary sinus was most commonly involved sinus in 18 of 30 patients (60%). Of them, eight were suspected to have allergic fungal sinusitis and were requested to undergo the invasive procedure required to obtain the pathological material for culture and histopathological examination. Parents of three children consented and AAS was documented in all of them including the one we had reported previously [15]. ‘Allergic mucin’ as evidenced by hyperdense lesions in the PNS on CT-PNS (Fig. 3A) were seen in all the 3 children with AAS. Histopathological finding in these patients included edematous polyps with eosinophilic infiltration (Fig. 3B). Fungal hyphae were demonstrated in the necrosed material using Gomori's methamine silver stain (Fig. 3C). The necrotic debris from the PNS cultured A. flavus in 2 of 3 and A. fumigatus in one. Only 1 of the 42 patients was suspected to have CF. On evaluation, the sweat chloride test was negative [14].
Fig. 3. (A) Computed tomography of the paranasal sinus showing hyperdense lesion in the left maxillary sinus suggestive of “allergic mucin.” (B) High power view (×400) on haematoxylin and eosin stain showing inflammation of the nasal mucosa consisting of eosinophils, neutrophils and histiocytes. (C) High power view (×400) on Gomori methamine silver stain showing branching septate hyphae of Aspergillus.
DISCUSSION
This study endeavored to retrospectively review 42 pediatric patients comprising 12% (42 of 349) of the ABPA patients diagnosed over a period of 31 years in one unit. Although ABPA has been fairly well documented in the adult population [27,28], only sporadic reports have described the clinical presentation of non-CF ABPA in the pediatric age group [6,7,8,9,12]. The first detailed description of ABPA in children was by Mann and Pasha [3] in 1959. The authors reported a 15 and half-year-old boy with allergic pulmonary aspergillosis and “Schonlein-Henoch” purpura. Wang et al. [9] documented 12 pediatric patients with ABPA. In 1982, Chetty et al. [29] documented the first 2 pediatric patients with ABPA in India. In a subsequent study from the same group, of the 107 patients with perennial asthma, ABPA was diagnosed in 35 who fulfilled four or more of the Rosenberg and Patterson criteria [10]. The major limitations of this study were that neither specific IgE/IgG levels were determined nor an attempt was made to demonstrate central bronchiectasis in these patients. In a recent prospective study from India, ABPA was documented in 26% of children with poorly controlled asthma [11]. In the adult patients with asthma from India, ABPA has been reported in 7.6% [27].
In patients with CF, studies from Europe and United States have documented a prevalence of ABPA in the range of 2%–15% [4,30]. However, CF is uncommon in the Indian population with about 500 patients being documented till 2015 [31,32]. In a series of 33 patients with CF from Delhi, ABPA was diagnosed in 6 (18.2%) [5].
ABPA has been documented in infants as young as 6 months of age [8]. In the first pediatric series of ABPA by Wang et al. [9], the mean age was 14.5 years. In the recent prospective study from India [11], the mean age on presentation was 9.56 ± 2.23 years with the youngest child being 5.5 years old. In our study, the mean age was 12.97 ± 4.02 years with the youngest being a 3.5-year-old child, who had been earlier reported for hilar lymphadenopathy [16]. A majority of our patients were males (male to female ratio, 1.62) as seen in previous reports [9,10,11].
All our patients were asthmatics with a family history of asthma present in 11 of 42 (26.2%) of our patients including 3 pairs of familial ABPA. We had previously reported the familial occurrence of ABPA to be 4.9% [17]. Based on the clinical and radiological profiles, a third of our patients had a prior history of antituberculous intake. In high tuberculous prevalent areas, due to the striking radiological similarities, ABPA is often mistaken for pulmonary tuberculosis. These patients receive antituberculous treatment for long duration without relief and are often labeled as drug resistant tuberculosis, despite lack of microbiological evidence.
In the prospective study among pediatric asthma patients from India [11], ABPA-S was diagnosed in 5 of 26 patients (19.2%) with a mean serum IgE being 5,376 ± 4,051 IU/mL. This study suggested 1,200 IU/mL as a cutoff value of serum IgE levels for the diagnosis of ABPA with a sensitivity and specificity of 88.5% and 70.5%, respectively. In 10 of our 42 patients, a diagnosis of ABPA-S was made. In 34 of 42 of our patients, serum IgE levels were higher than 417 kUA/L, the cutoff value proposed in the minimal essential criteria [33] however, the mean serum total IgE values were 4,509.16 ± 7,907.77 kUA/L.
Radiologically, ABPA is characterized by ‘transient pulmonary infiltrates’ or ‘fleeting shadows’ on serial roentgenograms [1]. Hinson et al. [2] were also the first to recognize this distinctive feature of ABPA. This characteristic radiological feature was documented in a third of our patients similar to that reported by Chetty et al. [10]. These “fleeting shadows” are usually observed in acute or exacerbation stage and reflect the ongoing disease process. These occur due to mucoid impaction in the damaged bronchi [34]. “Gloved-finger” appearance (Fig. 4A) due to mucoid impaction is peculiar to ABPA. This is a transient feature due to the mucoid impaction in the distal bronchus and may disappear as a result of coughing or therapy. Lobar/segmental collapse is not uncommon and this too is caused due to mucoid impaction. MLS due to chronic/recurrent collapse of the middle lobe has been described in patients with ABPA both in the adult [24] as well as pediatric population [18]. Although rare, the presence of true hilar adenopathy has been reported earlier in adults [35]. True hilar adenopathy in children with ABPA was first documented in a 42-month-old child who also happened to be the youngest patient in our series [16]. In 7 of our 42 patients (16.7%), we found either hilar, pretracheal or paratracheal lymphadenopathy. This highlights the need to consider ABPA in the differential diagnosis of thoracic lymphadenopathy.
Fig. 4. (A) Chest radiograph of a 16-year-old girl showing “gloved finger” appearance in the right upper zone (white arrow). (B) High resolution computed tomography of the chest (mediastinal window) showing high attenuation mucus. (C) High resolution computed tomography of the chest (lung window) showing bilateral lower lobe consolidation with air-bronchograms.
In 1967, Scadding [36] was the first to describe proximal/central bronchiectasis with normal peripheral tapering of the bronchus, a radiological feature pathognomic of ABPA. This continues to remain as a sine qua non for the diagnosis of ABPA-CB [1,34]. Bronchography, once considered to be the “gold standard” to demonstrate CB, was infrequently used in the pediatric age group due to safety issues [13]. With the advent of CT, bronchography is no longer in vogue and is now considered to be obsolete. CB was seen in 32 of our 42 patients (76.2%) which was similar to the observations by Wang et al. [9] where 7 out of 8 patients (87.5%) had CB. In contrast the recent prospective study from India, CB was demonstrated in 3 of 26 patients (11.5%) [11]. High attenuation mucus [37], considered to be a characteristic feature of ABPA, was documented in 7 of our 36 patients (19.4%). In a study among 155 adult patients with ABPA, HAM on HRCT (Fig. 4B) was reported in 18. These patients had higher serum total IgE and Aspergillus specific IgE with increased frequency of relapse [37]. CT findings such as mucoid impaction were seen in 16 of 36 patients while collapse and consolidation (Fig. 4C) were documented in 2 patients each. ABPA presenting as MLS in the pediatric age group has also been documented [18].
Of the 20 patients with radiological sinusitis, AAS was suspected in eight. Consent for the invasive procedure was obtained from only 3 parents. The diagnosis of AAS was established in all these three patients with ABPA. The radiological finding suggestive of AAS includes hyperdense lesions, ‘star-filled sky’ or ‘ground glass’ pattern. These occur due to the presence of high-density mineral element produced by the fungal elements [38]. The occurrence of upper airways symptoms and sinusitis has been documented in children with asthma [39]. However, in the pediatric patients with ABPA this is yet to be highlighted. Though allergic fungal sinusitis has been described in the pediatric population [40], its association with ABPA is not well defined. Campbell et al. [40] had reported the occurrence of allergic fungal sinusitis in children with asthma however, these patients had not been worked up for ABPA. The concomitant occurrence of ABPA with AAS, a clinical entity well characterized in adult patients, has been previously reported only once before in the pediatric age group [15].
Although the prevalence of ABPA in children with CF is well documented, the occurrence of this perplexing clinical entity in children with asthma is yet to receive the recognition it deserves. This study highlights the need to evaluate children with asthma for ABPA and to exclude AAS in those with upper airways symptoms. It is imperative to make an early diagnosis in these children so as to prevent long term morbidity caused by irreversible fibrotic changes that occurs with uncontrolled ABPA.
ACKNOWLEDGEMENTS
The authors thank Dr. Sudhir Jain, Senior Consultant Pathologist, for the photomicrographs.
References
- 1.Shah A, Panjabi C. Allergic bronchopulmonary aspergillosis: a perplexing clinical entity. Allergy Asthma Immunol Res. 2016;8:282–297. doi: 10.4168/aair.2016.8.4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinson KF, Moon AJ, Plummer NS. Broncho-pulmonary aspergillosis; a review and a report of eight new cases. Thorax. 1952;7:317–333. doi: 10.1136/thx.7.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann B, Pasha MA. Allergic primary pulmonary aspergillosis and Schonlein-Henoch purpura. Br Med J. 1959;1:282–283. doi: 10.1136/bmj.1.5117.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geller DE, Kaplowitz H, Light MJ, Colin AA. Allergic bronchopulmonary aspergillosis in cystic fibrosis: reported prevalence, regional distribution, and patient characteristics. Scientific Advisory Group, Investigators, and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Chest. 1999;116:639–646. doi: 10.1378/chest.116.3.639. [DOI] [PubMed] [Google Scholar]
- 5.Sharma VK, Raj D, Xess I, Lodha R, Kabra SK. Prevalence and risk factors for allergic bronchopulmonary aspergillosis in Indian children with cystic fibrosis. Indian Pediatr. 2014;51:295–297. [PubMed] [Google Scholar]
- 6.Slavin RG, Laird TS, Cherry JD. Allergic bronchopulmonary aspergillosis in a child. J Pediatr. 1970;76:416–421. doi: 10.1016/s0022-3476(70)80482-6. [DOI] [PubMed] [Google Scholar]
- 7.Berger I, Phillips WL, Shenker IR. Pulmonary aspergillosis in childhood A case report and discussion. Clin Pediatr (Phila) 1972;11:178–182. doi: 10.1177/000992287201100312. [DOI] [PubMed] [Google Scholar]
- 8.Imbeau SA, Cohen M, Reed CE. Allergic bronchopulmonary aspergillosis in infants. Am J Dis Child. 1977;131:1127–1130. doi: 10.1001/archpedi.1977.02120230073013. [DOI] [PubMed] [Google Scholar]
- 9.Wang JL, Patterson R, Mintzer R, Roberts M, Rosenberg M. Allergic bronchopulmonary aspergillosis in pediatric practice. J Pediatr. 1979;94:376–381. doi: 10.1016/s0022-3476(79)80574-0. [DOI] [PubMed] [Google Scholar]
- 10.Chetty A, Bhargava S, Jain RK. Allergic bronchopulmonary aspergillosis in Indian children with bronchial asthma. Ann Allergy. 1985;54:46–49. [PubMed] [Google Scholar]
- 11.Singh M, Das S, Chauhan A, Paul N, Sodhi KS, Mathew J, Chakrabarti A. The diagnostic criteria for allergic bronchopulmonary aspergillosis in children with poorly controlled asthma need to be re-evaluated. Acta Paediatr. 2015;104:e206–e209. doi: 10.1111/apa.12930. [DOI] [PubMed] [Google Scholar]
- 12.De H, Azad SM, Giri PP, Pal P, Ghosh A, Maitra A. Two cases of non-cystic fibrosis (CF) bronchiectasis with allergic bronchopulmonary aspergillosis. Respir Med Case Rep. 2016;20:68–71. doi: 10.1016/j.rmcr.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah A, Pant CS, Bhagat R, Panchal N. CT in childhood allergic bronchopulmonary aspergillosis. Pediatr Radiol. 1992;22:227–228. doi: 10.1007/BF02012505. [DOI] [PubMed] [Google Scholar]
- 14.Shah A, Bhagat R, Panchal N. Allergic bronchopulmonary aspergillosis with clubbing and cavitation. Indian Pediatr. 1993;30:248–251. [PubMed] [Google Scholar]
- 15.Shah A, Panchal N, Agarwal AK. Concomitant allergic bronchopulmonary aspergillosis and allergic Aspergillus sinusitis: a review of an uncommon association*. Clin Exp Allergy. 2001;31:1896–1905. doi: 10.1046/j.1365-2222.2001.01159.x. [DOI] [PubMed] [Google Scholar]
- 16.Shah A, Kala J, Sahay S. Allergic bronchopulmonary aspergillosis with hilar adenopathy in a 42-month-old boy. Pediatr Pulmonol. 2007;42:747–748. doi: 10.1002/ppul.20639. [DOI] [PubMed] [Google Scholar]
- 17.Shah A, Kala J, Sahay S, Panjabi C. Frequency of familial occurrence in 164 patients with allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol. 2008;101:363–369. doi: 10.1016/S1081-1206(10)60311-0. [DOI] [PubMed] [Google Scholar]
- 18.Shah A, Gera K, Panjabi C. Childhood allergic bronchopulmonary aspergillosis presenting as a middle lobe syndrome. Asia Pac Allergy. 2016;6:67–69. doi: 10.5415/apallergy.2016.6.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg M, Patterson R, Mintzer R, Cooper BJ, Roberts M, Harris KE. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med. 1977;86:405–414. doi: 10.7326/0003-4819-86-4-405. [DOI] [PubMed] [Google Scholar]
- 20.Wang JL, Patterson R, Rosenberg M, Roberts M, Cooper BJ. Serum IgE and IgG antibody activity against Aspergillus fumigatus as a diagnostic aid in allergic bronchopulmonary aspergillosis. Am Rev Respir Dis. 1978;117:917–927. doi: 10.1164/arrd.1978.117.5.917. [DOI] [PubMed] [Google Scholar]
- 21.Shah A, Khan ZU, Chaturvedi S, Malik GB, Randhawa HS. Concomitant allergic Aspergillus sinusitis and allergic bronchopulmonary aspergillosis associated with familial occurrence of allergic bronchopulmonary aspergillosis. Ann Allergy. 1990;64:507–512. [PubMed] [Google Scholar]
- 22.Shah A, Khan ZU, Sircar M, Chaturvedi S, Malik GB, Randhawa HS. Allergic Aspergillus sinusitis: an Indian report. Respir Med. 1990;84:249–251. doi: 10.1016/s0954-6111(08)80044-3. [DOI] [PubMed] [Google Scholar]
- 23.Bhagat R, Shah A, Jaggi OP, Khan ZU. Concomitant allergic bronchopulmonary aspergillosis and allergic Aspergillus sinusitis with an operated aspergilloma. J Allergy Clin Immunol. 1993;91:1094–1096. doi: 10.1016/0091-6749(93)90224-4. [DOI] [PubMed] [Google Scholar]
- 24.Shah A, Bhagat R, Panchal N, Jaggi OP, Khan ZU. Allergic bronchopulmonary aspergillosis with middle lobe syndrome and allergic Aspergillus sinusitis. Eur Respir J. 1993;6:917–918. [PubMed] [Google Scholar]
- 25.deShazo RD, Swain RE. Diagnostic criteria for allergic fungal sinusitis. J Allergy Clin Immunol. 1995;96:24–35. doi: 10.1016/s0091-6749(95)70029-3. [DOI] [PubMed] [Google Scholar]
- 26.Shah A. Allergic bronchopulmonary and sinus aspergillosis: the co-occurrence. Chest (India) 2001;2:234–235. [Google Scholar]
- 27.Maurya V, Gugnani HC, Sarma PU, Madan T, Shah A. Sensitization to Aspergillus antigens and occurrence of allergic bronchopulmonary aspergillosis in patients with asthma. Chest. 2005;127:1252–1259. doi: 10.1378/chest.127.4.1252. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal R, Gupta D, Aggarwal AN, Behera D, Jindal SK. Allergic bronchopulmonary aspergillosis: lessons from 126 patients attending a chest clinic in north India. Chest. 2006;130:442–448. doi: 10.1378/chest.130.2.442. [DOI] [PubMed] [Google Scholar]
- 29.Chetty A, Menon RK, Malviya AN. Allergic bronchopulmon ary aspergillosis in children. Indian J Pediatr. 1982;49:203–205. doi: 10.1007/BF02830750. [DOI] [PubMed] [Google Scholar]
- 30.Mastella G, Rainisio M, Harms HK, Hodson ME, Koch C, Navarro J, Strandvik B, McKenzie SG. Allergic bronchopulmonary aspergillosis in cystic fibrosis. Eur Respir J. 2001;17:1052–1053. doi: 10.1183/09031936.01.17510520. [DOI] [PubMed] [Google Scholar]
- 31.Kabra SK, Kabra M, Lodha R, Shastri S. Cystic fibrosis in India. Pediatr Pulmonol. 2007;42:1087–1094. doi: 10.1002/ppul.20677. [DOI] [PubMed] [Google Scholar]
- 32.Mandal A, Kabra SK, Lodha R. Cystic fibrosis in India: past, present and future. J Pulm Med Respir Res. 2015;1:002. [Google Scholar]
- 33.Greenberger PA. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 2002;110:685–692. doi: 10.1067/mai.2002.130179. [DOI] [PubMed] [Google Scholar]
- 34.Shah A, Panjabi C. Allergic aspergillosis of the respiratory tract. Eur Respir Rev. 2014;23:8–29. doi: 10.1183/09059180.00007413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah A, Agarwal AK, Chugh IM. Hilar adenopathy in allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol. 1999;82:504–506. doi: 10.1016/S1081-1206(10)62729-9. [DOI] [PubMed] [Google Scholar]
- 36.Scadding JG. The bronchi in allergic aspergillosis. Scand J Respir Dis. 1967;48:372–377. [Google Scholar]
- 37.Agarwal R, Gupta D, Aggarwal AN, Saxena AK, Chakrabarti A, Jindal SK. Clinical significance of hyperattenuating mucoid impaction in allergic bronchopulmonary aspergillosis: an analysis of 155 patients. Chest. 2007;132:1183–1190. doi: 10.1378/chest.07-0808. [DOI] [PubMed] [Google Scholar]
- 38.Panjabi C, Shah A. Allergic Aspergillus sinusitis and its association with allergic bronchopulmonary aspergillosis. Asia Pac Allergy. 2011;1:130–137. doi: 10.5415/apallergy.2011.1.3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mir E, Panjabi C, Shah A. Impact of allergic rhinitis in school going children. Asia Pac Allergy. 2012;2:93–100. doi: 10.5415/apallergy.2012.2.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell JM, Graham M, Gray HC, Bower C, Blaiss MS, Jones SM. Allergic fungal sinusitis in children. Ann Allergy Asthma Immunol. 2006;96:286–290. doi: 10.1016/S1081-1206(10)61237-9. [DOI] [PubMed] [Google Scholar]