Figure 2.
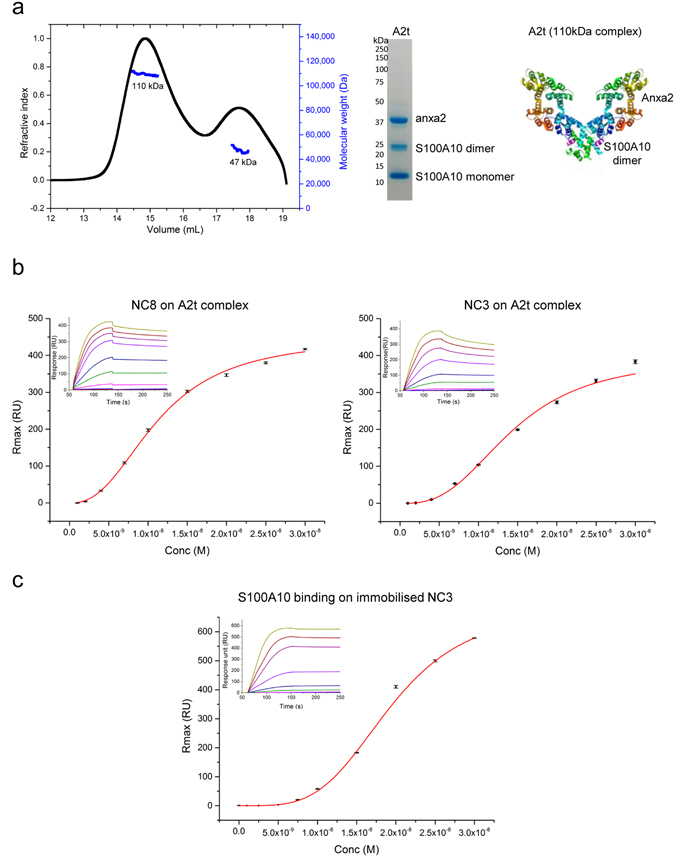
PLA2R interacts with A2t complex via S100A10. (a) Left, characterisation of Annexin2-S100A10 complex in solution using Multi Angle Laser Light Scattering (MALLS). The elution profile reveals 2 peaks, 110 kDa referred as A2t (Anxa2/S100A10 heterotetramer) and 47 kDa complex. Middle, 10 µg of purified complex ran on a non-reduced SDS-PAGE gel and stained with Instant Blue confirm the presence of both Anxa2 and S100A10 proteins in various oligomeric forms. Right, schematic representation of A2t complex made of a S100A10 dimer (10.2210/pdb1BT6/pdb) and two Anxa2 molecules (10.2210/pdb2hyw/pdb). (b) SPR data showing direct interaction with PLA2R fragments and A2t complex. Purified PLA2R NC8 and NC3 at various concentrations (0 to 300 nM) were injected over the surfaces of immobilised A2t. The maximal response (RUmax) were obtained from the sensograms (inset) and plotted against the increasing concentrations of injected PLA2R. Each data point in both experiments is the mean of 3 repeats. The equilibrium dissociation constant, K D, was calculated by fitting the data to the Hill equation using nonlinear regression. (c) Representative sensorgrams derived from injections of different concentrations of purified recombinant S100A10 protein over immobilised PLA2R NC3. Results were obtained after reference subtraction.
