Figure 1.
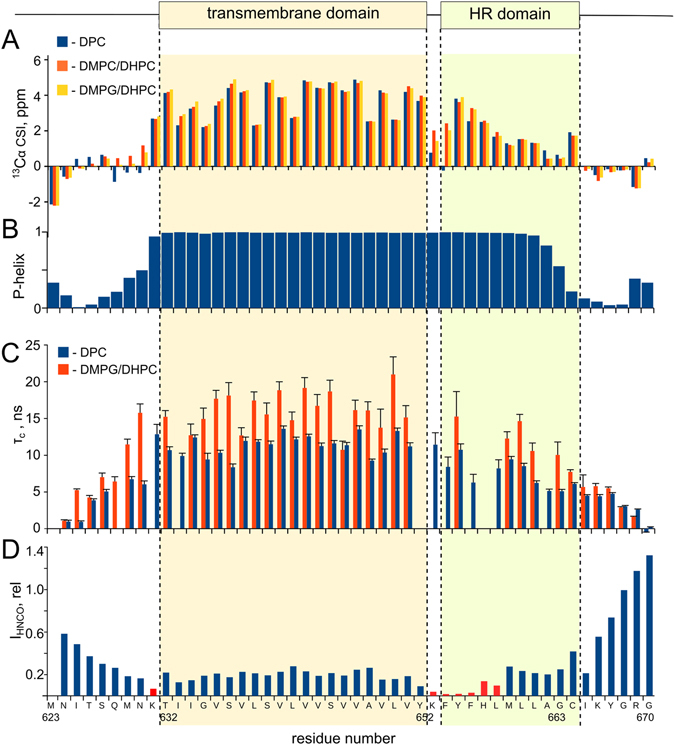
Secondary structure and dynamics of TLR4-TMICL. (A) Secondary chemical shifts of Cα nuclei of TLR4-TMICL in the environment of DPC micelles (blue bars), DMPC/DHPC q = 0.4 bicelles (red bars) and DMPG/DHPC q = 0.4 bicelles (yellow bars). Positive values indicate the helical conformation. (B) Propensity of helical secondary structure for the residues of TLR4-TMICL in DMPG/DHPC bicelles, according to the chemical shift based prediction by TALOS-N52. (C) Correlation time of rotational diffusion measured for the individual amide groups of TLR4-TMICL in DMPG/DHPC q = 0.4 bicelles (red bars) and DPC micelles (blue bars). Correlation times were measured from the rates of cross-correlated relaxation55. (D) Cross-peak intensities in the 3D HNCO spectrum of TLR4-TMICL in DMPG/DHPC q = 0.4 bicelles, expressed in relative units. Low intensity indicates the line broadening, enhanced transverse relaxation and motions in us-ms timescale. On top, the predicted TM domain (according to UniProt) and hydrophobic part of the ICL region (HR domain), homologous to the reported in ref. 23 are indicated.
