Figure 6.
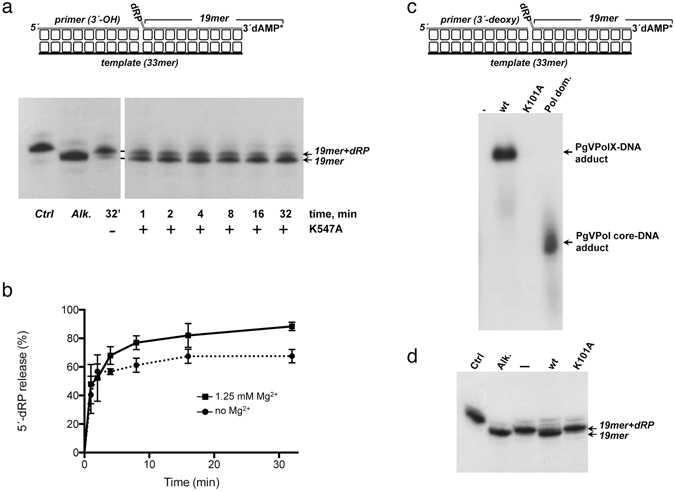
5′-dRP lyase activity of PgVPolX. (a) Time course analysis of the 5′-dRP release by PgVPolX. The assay was performed as described in Materials and Methods with 1 nM of the depicted substrate (top), and 3 nM of the ligase deficient-mutant K547A. After incubation for the indicated times, samples were analyzed by 7 M urea-20% PAGE and further autoradiography. Position of products is indicated. Alk. Alkalyne hydrolysis of the 5′-dRP moiety. (b) Quantification of the 5′-dRP released by PgVPolX. The assay was performed as in (a) with 1 nM of the depicted substrate (top), 3 nM of the ligase-deficient mutant K547A in the absence or presence of 1.25 mM MgCl2. The values plotted represent the ratio 19mer/(19mer +19mer +dRP) ×100 at each reaction time and are the mean of three independent experiments. (c) Formation of PgVPolX-DNA adducts. Reactions were performed as described in Materials and Methods, incubating 12 nM of the indicated protein, 6 nM of the depicted [32P]3′-labeled DNA substrate (top) and 100 mM NaBH4. Samples were analyzed by 10% SDS-PAGE and further autoradiography. (d) 5′-dRP lyase activity of PgVPolX relies on residue Lys101. The assay was performed as described in Materials and Methods by incubating 4 nM of the depicted substrate (top of panel c) and 3 nM of the indicated protein. After incubation for 2.5 min, samples were analyzed by 7 M urea-20% PAGE and further autoradiography. Alk. Alkalyne hydrolysis of the 5′-dRP moiety.
