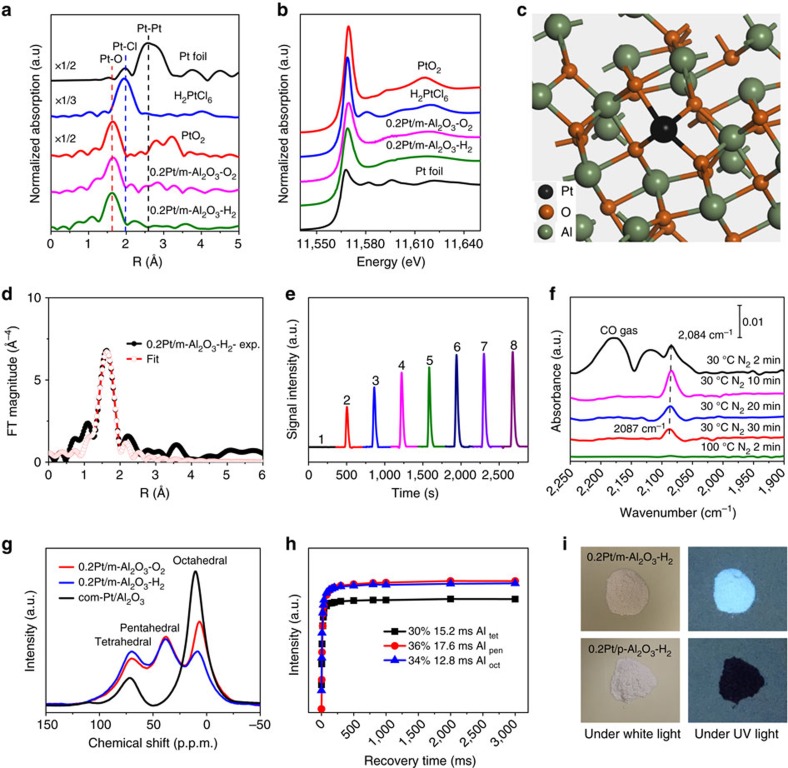
Figure 3. Characterization of the Pt single-atom and other control samples.
(a) The k3-weighted Fourier transform of EXAFS spectra derived from EXAFS, ▵=3.0–12.0 Å−1, (b) normalized XANES spectra at the Pt L3-edge of Pt foil, H2PtCl6, and PtO2, 0.2Pt/m-Al2O3-O2 and 0.2Pt/m-Al2O3-H2, (c) schematic illustration of individual Pt atom located on the surface of m-Al2O3 for sample 0.2Pt/m-Al2O3-H2, (d) FT-EXAFS curves between the experimental data and the fit, (e) H2-O2 titration profiles, (f) IR spectra of CO adsorbed after the desorption processes for 0.2Pt/m-Al2O3-H2, (g) the 27Al MAS-NMR spectra of 0.2Pt/m-Al2O3-O2, 0.2Pt/m-Al2O3-H2, and commercial Pt/Al2O3, (h) relative intensity changes of tetra-, penta- and octa-coordinated Al2O3 with recovery time for a spin-lattice relaxation measurement of Al2O3 for 0.2Pt/m-Al2O3-H2, (i) photographs of 0.2Pt/m-Al2O3-H2 and 0.2Pt/p-Al2O3-H2 under visible light and UV light (365 nm).