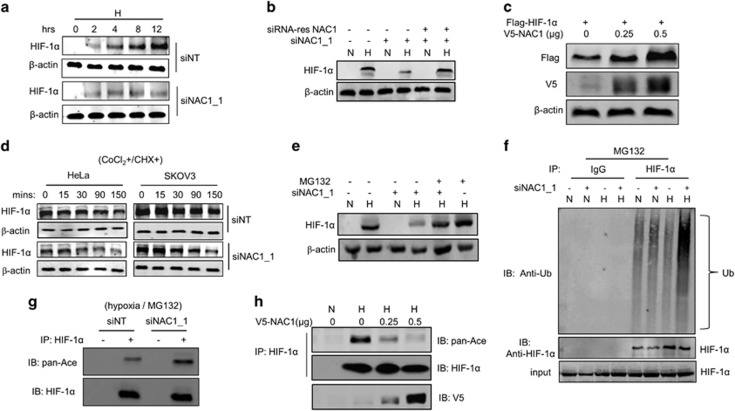
Figure 2.
NAC1 promotes stabilization of HIF-1α protein. HeLa cells were transfected with a siNT or NAC1 siRNA, then: (a) exposed to hypoxia for different periods of time. HIF-1α protein was determined by western blot; (b) followed by transfection with a siRNA-resistant NAC1-expressing plasmid or empty vector. The cells were then exposed to hypoxia for 24 h. HIF-1α protein was determined by western blot. β-Actin was used as a loading control. (c) HEK293T cells were co-transfected with a V5-NAC1 plasmid (0.25 and 0.5 μg) and a Flag-HIF-1α plasmid for 48 h, and then HIF-1α (Flag) and NAC1 (V5) protein were examined by western blot. (d) HeLa and SKOV3 cells with or without silencing of NAC1 expression were treated with CoCl2 for 6 h, and then pulse-chased in the presence of cycloheximide (15 μg/ml). HIF-1α protein was determined by western blot. (e) HeLa cells with or without silencing of NAC1 expression were incubated under normoxia or hypoxia for 24 h, and then treated with vehicle or 5 μM MG132 for 6 h. HIF-1α protein was examined by western blot. (f) HeLa cells with or without silencing of NAC1 expression were incubated under normoxia or hypoxia for 24 h, and treated with vehicle or 5 μM MG132 for 6 h. Cell lysates were immunoprecipitated with a control IgG or anti-HIF-1α antibody. The immunoprecipitates and input were probed for Ub and HIF-1α by immunoblotting. (g) HeLa cells with or without silencing of NAC1 expression were incubated under hypoxia for 24 h, and treated with 5 μM MG132 for another 6 h. Cell lysates were prepared and immunoprecipitated with an anti-HIF-1α antibody, and immunoblotted for HIF-1α and its acetylation form (pan-Ace). (h) HEK293T cells were transfected with a V5-NAC1 plasmid (0.25 and 0.5 μg) or a control plasmid for 24 h, and incubated under normoxia or hypoxia for additional 12 h. Cell lysates were immunoprecipitated with an anti-HIF-1α antibody, and then immunoblotted for HIF-1α and its acetylation form (pan-Ace).