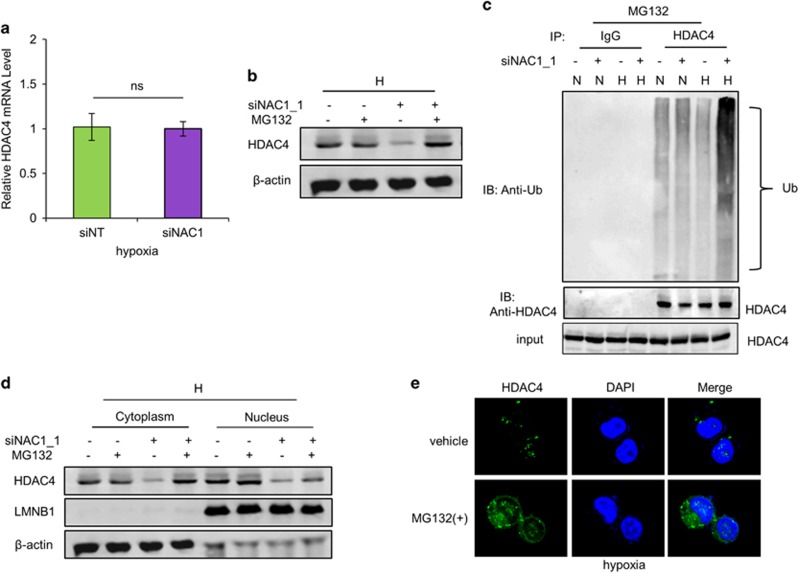
Figure 4.
NAC1 regulates subcellular localization and degradation of HDAC4 in hypoxic cells. (a) HeLa cells were transfected with a siNT or NAC1 siRNA, followed by exposure to hypoxia for 24 h. HDAC4 mRNA was measured by quantitative reverse transcriptase (RT)–PCR, and plotted after normalization. Bars are mean±s.d. (n=3). (b) HeLa cells with or without silencing of NAC1 were exposed to hypoxia for 24 h, and treated with vehicle or 5 μM MG132 for another 6 h. HDAC4 protein was examined by western blot. (c) HeLa cells with or without silencing of NAC1 expression were incubated under normoxia or hypoxia for 24 h, and treated with 5 μM MG132 for another 6 h. Cell lysates were immunoprecipitated with a control IgG or anti-HDAC4 antibody, followed by immunoblotting for Ub. (d, e) HeLa cells with or without silencing of NAC1 expression were incubated under hypoxic condition for 24 h, and then treated with vehicle or 5 μM MG132 for another 6 h. (d) HDAC4 protein in the nuclear and cytoplasmic fractions was detected by western blot. β-Actin, a loading control for the cytoplasmic fraction; LMNB1, loading control for the nuclear fraction. (e) Immunofluorescent detection of HDAC4 was carried out using an anti-HDAC4 antibody and Alexa Fluor 488 goat anti-rabbit secondary antibody (green). Blue, cells were counterstained with 4,6-diamidino-2-phenylindole (DAPI).