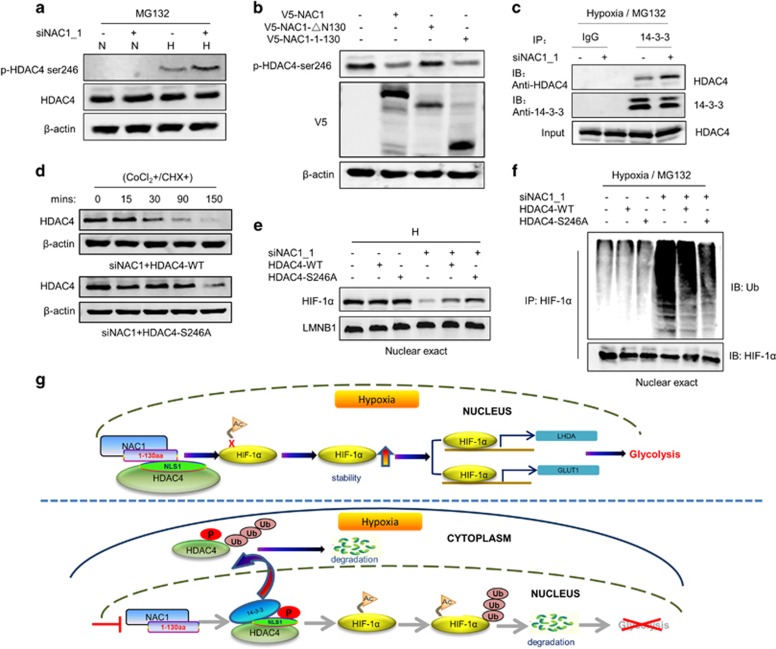
Figure 6.
NAC1-HDAC4 binding interferes with S246 phosphorylation of HDAC4. (a) HeLa cells were transfected with a siNT or NAC1 siRNA, and then incubated under normoxia or hypoxia for 24 h, followed by treatment with vehicle or 5 μM MG132 for another 6 h. The phosphorylation of HDAC4 was detected by western blot using an anti-phospho-HDAC4 (Ser246) antibody. (b) ES-2 cells transfected with V5-NAC1, V5-NAC1-ΔN130 or V5-NAC1-1-130 plasmids were exposed to hypoxia for 24 h. The phosphorylation of HDAC4 was detected by western blot. (c) HeLa cells were transfected with an siNT or NAC1 siRNA, and incubated under hypoxia for 24 h, followed by treatment with vehicle or 5 μM MG132 for another 6 h. Lysates of the treated cells were immunoprecipitated with a control IgG or anti-14-3-3 antibody, and the immunoprecipitates were analyzed for HDAC4 by western blot. (d) HeLa cells with silencing of NAC1 expression were transfected with a wild-type Flag-HDAC4 plasmid or a phosphorylation mutant, Flag-HDAC4 (S246A). Twenty-four hours later, the cells were treated with CoCl2 for 6 h, and then pulse-chased for HDAC4 protein in the presence of cycloheximide (15 μg/ml). HDAC4 protein was detected by western blot. (e, f) HeLa cells with or without silencing of NAC1 expression were transfected with the Flag-HDAC4 (WT) or Flag-HDAC4 (S246A) plasmid for 12 h, and exposed to hypoxia for additional 24 h. (e) Nuclear HIF-1α was detected by western blot, with LMNB1 as a loading control for the nuclear fraction. (f) Following 6-h treatment with 5 μM MG132, nuclear extracts were prepared and immunoprecipitated with an anti-HIF-1α antibody. The immunoprecipitates were then subjected to immunoblotting for Ub. (g) Proposed model for the role of NAC1-HDAC4-HIF-1α in promoting glycolysis in hypoxic cells.