Figure 5.
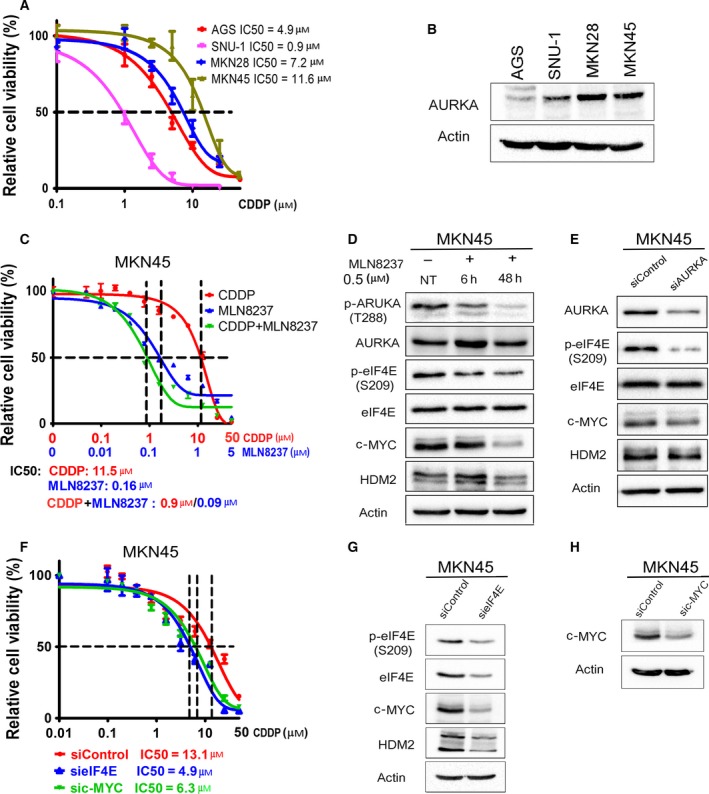
AURKA mediates de novo CDDP resistance through regulation of eIF4E and its downstream effectors. (A) Gastric cancer cells (AGS, SNU‐1, MKN28, and MKN45) in 96‐well plates were treated with CDDP following an 8‐point twofold serial dilution, for 5 days. Cells were then subjected to CellTiter‐Glo assay to determine cell viability. IC50 values were determined as described in Materials and methods. (B) Protein extracts from the gastric cancer cell lines, shown in panel A, were subjected to western blot analysis of the indicated proteins. The results indicated high protein levels of AURKA in cell lines with de novo CDDP resistance (MKN28 and MKN45). (C) CDDP‐resistant MKN45 cells in 96‐well plates were treated with CDDP, MLN8237, or CDDP + MLN8237 at a fixed ratio (10 : 1) following a 12‐point twofold serial dilution of CDDP, for 5 days. The CellTiter‐Glo assay results showed that treatment with MLN8237 alone or in combination with CDDP significantly reduced cell viability in comparison with the treatment with CDDP alone (P < 0.001). (D) MKN45 cells were treated with MLN8237 (0.5 μm) for 6 h or 48 h. (E) MKN45 cells were transiently transfected with siControl or siAURKA for 48 h. Cell lysates were subjected to western blot analysis of the indicated proteins. The results indicated that pharmacologic inhibition (D) or knockdown (E) of AURKA reduces protein levels of p‐eIF4E (S209), c‐MYC, and HDM2 in CDDP‐resistant MKN45 cells. (F) MKN45 cells were transfected with siControl, sieIF4E, or sic‐MYC for 48 h and subjected to CellTiter‐Glo viability assay. Knocking down of eIF4E or c‐MYC significantly sensitized cells to CDDP (P < 0.05), as indicated by twofold decrease in CDDP IC50. (G,H) Cell lysates were subjected to western blot analysis of p‐eIF4E (S209), eIF4E, c‐MYC, and HDM2 proteins. Gel loading was normalized for equal β‐actin.
