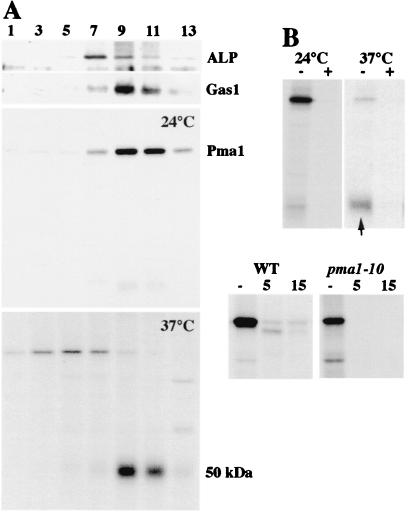
Figure 2.
Cell surface delivery of Pma1-10. (A) Density gradient fractionation. Cells (XGY32) were pulse-labeled for 5 min and chased for 30 min at 24°C or 37°C. Lysates were prepared and separated on Renografin density gradients as described in Materials and Methods. Pma1 was immunoprecipitated from gradient fractions. Distribution of ALP and Gas1 was determined by Western blotting gradient fractions. (B) Specificity of immunoprecipitation. Anti-Pma1 antibody was incubated in the presence (+) or absence (−) of 5 μg of partially purified Neurospora Pma1 (gift of Carolyn Slayman, Yale University) before adding to RIPA buffer containing peak plasma membrane fractions (9 and 10) from pulse–chase experiments in A. Arrow indicates 50-kDa fragment. (C) Limited trypsinolysis. Total membranes were prepared from cells pulse-labeled for 2 min at 37°C and incubated in the absence (−) and presence of trypsin at 30°C for 5 and 15 min, as described in Materials and Methods. Immunoprecipitated Pma1 was analyzed by SDS/PAGE and fluorography.