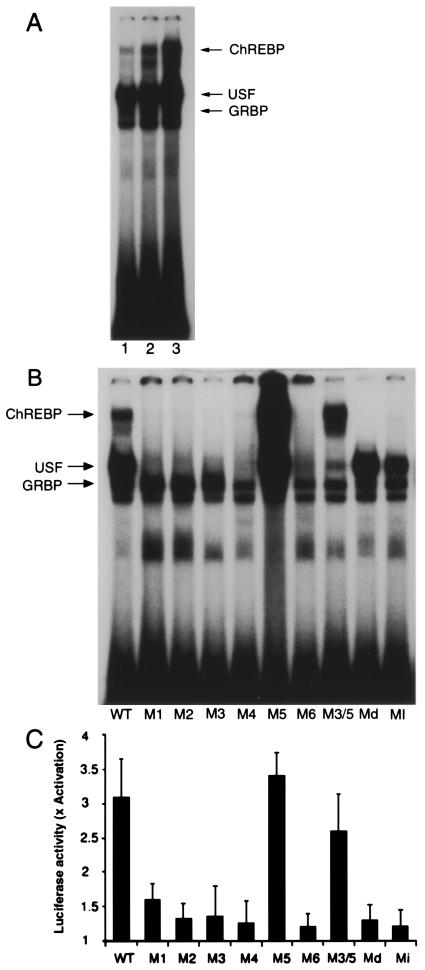
Figure 1.
(A) Gel-shift analysis of hepatic nuclear factors that recognize the WT LPK ChRE. Rats were starved for 48 h (lane 1) and subsequently re-fed a high-fat diet for 24 h (lane 2) or a high-carbohydrate diet for 24 h (lane 3). Nuclear extracts were prepared from the rat livers and incubated with a radiolabeled oligonucleotide corresponding to the WT LPK ChRE. Lanes 2 and 3 contain equal protein loads. Arrows indicate the positions of the DNA-binding complexes containing USF or the glucose response element binding protein (GRBP) as determined by supershift of the DNA-binding complex with antibodies specific for each protein. (B) Mutations to the LPK ChRE differentially affect the formation of the various DNA-binding complexes. The seuences of the mutated oligonucleotides are shown in Table 1 (Md and Mi correspond to S−1 and S+1, respectively). Liver nuclear extracts from rats r-fed a high-carbohydrate diet for 24 h after 48-h starvation were incubated with the WT and the mutated oligonucleotides and examined by gel-shift analysis. (C) Mutations to the LPK ChRE affect glucose-responsive transcription of a downstream luciferase reporter gene. Construction of luciferase reporters driven by the WT and variant LPK promoter sequences (Table 1) was described in ref. 13. Primary hepatocytes were transfected with the reporter constructs and the fold activation of luciferase expression in response to high glucose (27.5 mM) was determined. Values represent the mean ± standard error of four experiments.