Figure 7. Temperature promotes ATP‐independent endocytosis.
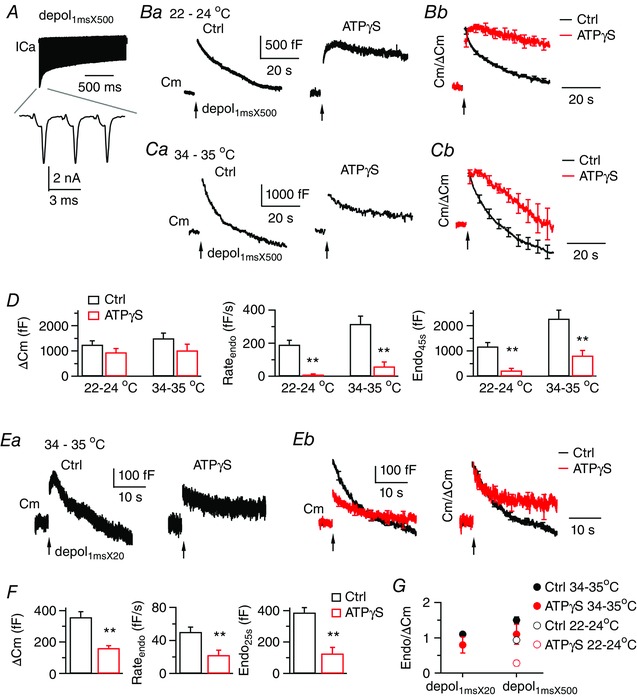
A, ICa currents induced by depol1msX500 from a control calyx, which was perfused by 22–24°C solution containing 2 mm Ca2+. The lower trace shows ICa induced by the first three pulses. B and C, sampled Cm responses (Ba and Ca) to depol1msX500 under dialysis with control or 4 mm ATPγS. Calyces were perfused by bath solution of 22–24°C or 34–35°C. Average Cm traces after normalization to ΔCm are superimposed in (B b) and (Cb). At calyces dialysed with ATPγS, depol1msX500 triggered a more significant amount of endocytosis at 34–35°C, which largely reset membrane expansion from exocytosis. D, ΔCm, Rateendo and Endo45s induced by depol1msX500 from control (n = 10) and ATPγS (n = 9) at 22–24°C, as well as control (n = 9) and ATPγS (n = 9) at 34–35°C. E, sampled Cm responses (Ea) induced by depol1msX20 from calyces perfused by solution of 34–35°C. The example for ATPγS represents intermediate inhibition of endocytosis. In some examples that are not shown, endocytosis was either almost fully blocked, or induced endocytosis overshoot. Average Cm traces without (Eb, left, 11 calyces for control, 10 calyces for ATPγS) and with normalization to ΔCm (Eb, right) are superimposed. Depol1msX20 triggered a significant component of ATP‐independent endocytosis. F, ΔCm, Rateendo and Endo45s induced by depol1msX20 from control (n = 11) and ATPγS (n = 10) at 34–35°C. G, Endo45s (depol1msX500) or Endo25s (depol1msX20) normalized to ΔCm. At 34–35°C, depol1msX500 and depol1msX20 induced significant ATP‐independent endocytosis to recover membrane close to the amount of exocytosis, resulting in a value near 1 for Endo/ΔCm. [Color figure can be viewed at wileyonlinelibrary.com]
