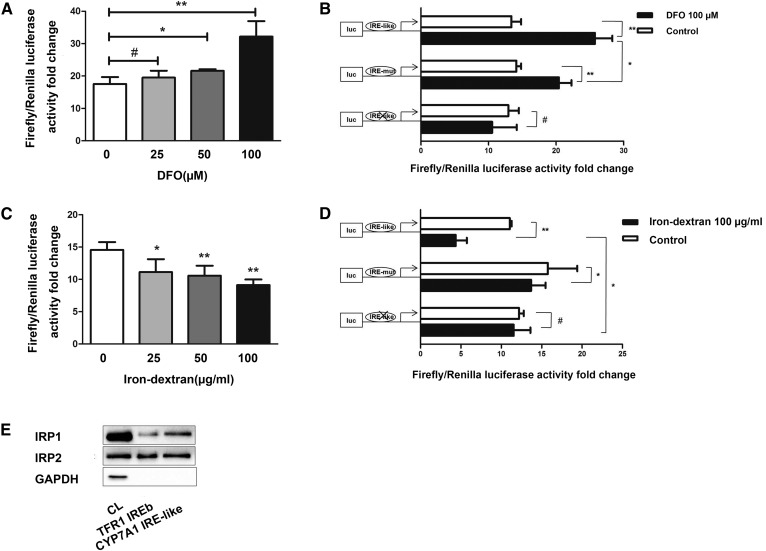
Fig. 5.
The IRE in the 3′-UTR of Cyp7a1 mRNA binds both to IRP1 and IRP2. A: TFR1 3′-UTR sequences inserted in the 3′-UTR of Luciferase reporter gene within the 3′-UTR HindIII/SpeI site. DNA from Luc/TFR1-3′-UTR-WT (100 ng) was transiently transfected into Hepa1-6 cells for 24 h as follows: untreated and DFO (0–100 μM) for 24 h prior. The TK plasmid was transfected to standardize. Luciferase activity of groups treated with DFO are higher than that of untreated group, and the highest activity was observed when 100 μM DFO was added. B: Luciferase reporter gene experiments performed in 100 μM DFO pretreated Hepa1-6 cells transfected with Luc/CYP7A1-3′-UTR-WT, Luc/CYP7A1-3′-UTR-MUT, and Luc/CYP7A1-3′UTR-CUT. The TK plasmid was transfected to standardize. C: DNA from Luc/TFR1-3′-UTR-WT (100 μg) was transiently transfected into Hepa1-6 cells for 24 h as follows: untreated and iron-dextran (0–100 μg/ml) for 24 h prior. The TK plasmid was transfected to standardize. Luciferase activity of groups treated with iron-dextran is lower than that of untreated group, and the lowest activity was observed when 100 μg/ml iron-dextran was added. D: Luciferase reporter gene experiments were performed in 100 μg/ml iron-dextran-pretreated Hepa1-6 cells transfected with Luc/CYP7A1-3′-UTR-WT, Luc/CYP7A1-3′-UTR-MUT, and Luc/CYP7A1-3′-UTR-CUT. The TK plasmid was transfected to standardize. E: Hepa1-6 cellular extracts were incubated with biotinylated RNA oligonucleotides of core IRE sequences from the TFR1 and Cyp7a1 mRNA 3′-UTRs. The protein-IRE complexes were precipitated with streptavidin-coated beads followed by Western blotting of IRP1/IRP2 proteins. GAPDH was used to exclude false positive reactions. #P > 0.05, *P < 0.05, **P < 0.01.