Abstract
Background:
Invasive fungal infection (IFI) is one of the leading causes of early death after renal transplantation. Voriconazole (VRC) is the first-line drug of IFI. Because of the large inter- and intraindividual variability in VRC plasma concentrations and the narrow therapeutic window for treating patients with IFIs, it is crucial to study the factors which could influence pharmacokinetic variability. We performed a population pharmacokinetics (PPK) study of VRC for personalized medicine.
Methods:
A total of 125 trough concentrations (Cmin) from 56 patients were evaluated, retrospectively. Nonlinear mixed effect model was used to describe a PPK model that was internally validated by bootstrap method. Potential covariates included demographic characteristics, physiological and pathological data, concomitant medications, and CYP2C19 genotype.
Results:
A 1-compartment model with first-order absorption and elimination was fit to characterize the VRC pharmacokinetics in renal transplant recipients (RTRs). Aspartate aminotransferase (AST) had a significant influence on clearance (CL) while CYP2C19 genotype had a major impact on the volume of distribution (V). The parameters of CL and V were 4.76 L/h and 22.47 L, respectively. The final model was V (L) = 22.47 × [1 + 2.21 × (EM = 1)] × [1 + 4.67 × (IM = 1)] × [1 + 3.30 × (PM = 1)] × exp (0.96); CL (L/h) = 4.76 × (AST/33)^(−0.23) × exp (0.14). VRC Cmin in intermediate metabolizers was significantly higher than in extensive metabolizers.
Conclusions:
Liver function and CYP2C19 polymorphism are major determinants of VRC pharmacokinetic variability in RTRs. Genotypes and clinical biomarkers can determine the initial scheme. Subsequently, therapeutic drug monitoring can optimize clinical efficacy and minimize toxicity. Hence, this is a feasible way to facilitate personalized medicine in RTRs. In addition, it is the first report about PPK of VRC in RTRs.
Key Words: voriconazole, population pharmacokinetics, renal transplant recipients, CYP2C19 genotype
INTRODUCTION
Kidney transplantation is the substitutive and lifesaving treatment for some individuals with end-stage renal disease, which can significantly prolong the survival time and improve the quality of life of uremia patients. Clinical studies showed that invasive fungal infection (IFI) is the second leading cause of early death in renal transplant recipients (RTRs), which is a consequence of the long-term use of immunosuppressants and broad-spectrum antibiotics. Despite its low incidence (1.3%),1 it has a high mortality rate (40%–60%) of IFI,2–4 especially in invasive pulmonary aspergillosis (81.3%). It has been reported that patients with IFI have an increased risk of delayed graft function and multiple infections.5
Voriconazole (VRC) is an azole compound with a broad-spectrum antifungal activity. Currently, it is the gold standard therapy of invasive aspergillosis,6 candidiasis,7 and other serious IFIs. It is recommended that VRC requires for therapy drug monitoring (TDM), due to its wide intra- and interindividual variability, narrow therapeutic range, and risk of toxicity including neurotoxicity (auditory hallucinations and hepatic encephalopathy), liver and visual toxicity.8–10 As a result, it is important to find crucial factors associated with pharmacokinetic variability of VRC.
To date, published data regarding RTRs taking VRC are limited to epidemiology, risk factors of infection,2–4,11 and effect on immunosuppressants.12,13 Research is mainly focused on hematopoietic stem cell transplantation, liver or lung transplantation, and intensive care patients taking VRC. However, the population pharmacokinetics (PPK) of VRC has not been reported in RTRs.
Accordingly, the aims of the current study were (1) to characterize the PPK model of VRC in RTRs and to explore the crucial factors influencing VRC serum concentration, and (2) to study distribution of CYP2C19 genotype in RTRs and its effect on VRC serum concentration.
MATERIALS AND METHODS
Patients and Methods
Patients and Data Collection
All patients with a history of renal transplantation who were admitted to the hospital and received VRC for the prevention or treatment of IFI from September 2015 to June 2016 were eligible for this study. This was a retrospective observational study at the Department of Urological Organ Transplantation of the Second Xiangya Hospital, Central South University. This study was approved by the Ethics Research Committee of the Second Xiangya Hospital, Central South University (yxlb-lcys-201501). Exclusion criteria were as follows: (1) age ≤15 years; (2) concomitant medications known to influence VRC pharmacokinetics (eg,, rifampin, a strong inducer of CYP2C19); and (3) patients with significant clinical data missing.
Patients' medical records were reviewed by using a standardized data collection template,14 including VRC dosing, frequency and duration of use, demographic information (age, sex, and weight), laboratory test results (blood, liver, and kidney function index), time after transplant, and concomitant medications taken during voriconazole therapy [glucocorticoid and proton pump inhibitors (PPI)]. A blood sample is obtained from each patient to determine the VRC Cmin and CYP2C19 gene phenotype (CYP2C19*2, CYP2C19*3, and CYP2C19*17).
Blood Sampling and Analytical Assays
The usual dose for VRC is 200 mg b.i.d. as starting dose. The doses were adjusted based on first Cmin. All Cmin were collected 30 minutes before the next dose. The VRC serum concentrations were analyzed by automatic 2-dimensional liquid chromatography (2D-HPLC; Dmitr Instrument Co Ltd, Hunan, China). Two-dimensional separation condition consists of the following: the first-dimensional chromatographic column was FRO C18 (100 × 3.0 mm, 5 μm, ANAX); mobile phase: 20 mmol/L ammonium acetate–acetonitrile = 48:52 (vol/vol); flow rate: 1.0 mL/min. The second-dimensional chromatographic column was ASTON HD C18 (150 × 4.6 mm i.d., 5 μm, ANAX); mobile phase: 40 mmol/L ammonium acetate–acetonitrile = 85:15 (vol/vol); flow rate: 1.2 mL/min; detection wavelength: 273 nm; column temperature: 45°C; sample size: 200 μL. The linearity range was 0.35–11.3 mcg/mL. The intraday and interday precisions were within 1.94%–2.22% and 2.15%–6.78%, separately. The absolute and relative recovery ranged from 88.2% to 93.6% and from 94.2% to 105.3%. The stability of blood sample at room temperature for 8 hours and in −20°C of 3 repeated freeze–thaw cycles were within ±8% and ±10%, respectively.
DNA Sequencing and CYP2C19 Genetic Polymorphism
CYP2C19 phenotypic subgroups were classified into 5 categories, namely: (1) ultrarapid metabolizer (UM, CYP2C19*17/*17), (2) rapid metabolizer (CYP2C19*1/*17), (3) extensive metabolizer (EM, CYP2C19*1/*1), (4) intermediate metabolizer (IM, CYP2C19*1/*2, CYP2C19*1/*3, CYP2C19*2/*17), and (5) poor metabolizer (PM, CYP2C19*2/*2, CYP2C19*2/*3, CYP2C19*3/*3).15
Blood samples (1–3 mL) for genotype detection were obtained from 56 patients. DNA was purified by using the E.Z.N.A SQ Blood DNA Kit II (Omega Bio-Tek, Norcross, GA) method. Genotype test adopted Sanger dideoxy DNA sequencing method by using ABI3730xl-full automatic sequencing instrument (ABI Co) from BoShang Biotechnology Co Ltd in Shanghai.
Population Pharmacokinetics Analysis
Nonlinear mixed effect model (NONMEM) was performed by using Phoenix NLME software (Version 1.4, Pharsight, A Certara Company, USA). Extended least square method (FOCE ELS) was adopted in the whole process. Kruskal–Wallis test was used to analyze the correlation of Cmin with dosage form and time after transplant (P < 0.05).
Structural Model
Nonlinear mixed-effects model to analyze PPK may offer the possibility of gaining information on pharmacokinetics from relatively sparse data. We use multiple-trough sampling (Cmin) method and NONMEM to estimate interindividual variability in clearance (CL) and volume of distribution (V), by fixing absorption rate constant (Ka).16 A study showed that a linear elimination model would appropriate to VRC serum concentration compared with other linear models.17 The 1-compartment model with first-order absorption and elimination was fitted with VRC pharmacokinetic model and the absorption rate was fixed to a value of 1.1 h.18–20
Statistical Model
The following exponential models were used to determine the interindividual variability:
 |
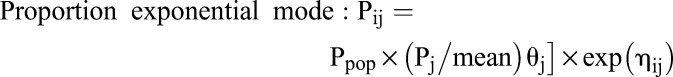 |
Residual variability was tested by comparing 4 models as follows:
The following variables were used for all equations: Pij is the pharmacokinetic parameter of a certain individual, Ppop is typical values of PPK parameters, Pj is covariate (influencing factor), θj is corrected value of covariate, ηij is individually random variation with a mean of zero and a variance of ω2; Cobs is observed concentrations, Cpred is predicted concentrations, and εi is random variation with a mean of zero and a variance of σ2. The Optimal model was determined by considering objective function value (OFV), coefficient of variation, and RetCode value.
Covariate Model
Firstly, the correlations between pharmacokinetic parameters and covariates were preliminarily inspected by the line graph. Then, covariates were incorporated in the base model, one at a time, using forward addition and backward deletion (stepwise). A significant covariate was retained when the following criteria were met: (1) a decrease in OFV >3.84 (P < 0.05) was included in forward addition, and an increase in OFV >6.63 (P < 0.01) was significant in backward deletion (approximate to χ2 distribution,  = 3.84,
= 3.84, 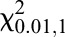 = 6.63), (2) clinical plausibility for added variable, and (3) the 95% confidence interval (CI) for parameter estimates did not include zero.
= 6.63), (2) clinical plausibility for added variable, and (3) the 95% confidence interval (CI) for parameter estimates did not include zero.
Model Selection and Validation
Goodness-of-fit statistics and graphical plots were used to evaluate the adequacy of fitting. Accuracy and stability of prediction of covariate model were validated by bootstrap method. One thousand resamples from the original date were performed. Mean values and 95% CI of bootstrap parameters were compared with estimates of the final model.
RESULTS
Patient Demographics and Dose Characteristics
A total of 125 Cmin (a median of 2.18, range = 0.16–9.59 mcg/mL) from 56 inpatients were collected. An average of 2–3 blood samples were collected from each patient. Patients' demographics and characteristics are summarized in Table 1. There was still a broad variation in Cmin of VRC under the monitoring. About 72.1% of Cmin values were in therapeutic window (1.0–5.5 mcg/mL), while 20.0% were subtherapeutic and 8.8% were supratherapeutic. Occurrence of IFI was likely in the first year after transplantation (78.6%), which is a serious threat for successful outcomes following kidney transplantation.
TABLE 1.
Demographic and Clinical Data

Population Pharmacokinetic Analysis
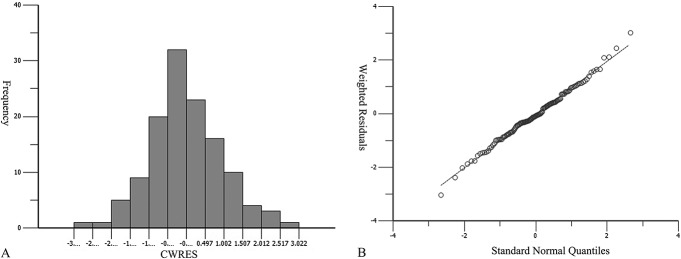
A 1-compartment model with first-order absorption and elimination was sufficient to characterize VRC pharmacokinetics. The population-typical values of CL and V were 4.76 L/h and 22.47 L, respectively. Interindividual variability was described by the proportion exponential model, and residual variability was described by proportional error model [Cobs = Cpred × (1 + 0.15)]. As shown in Figure 1, distribution of conditional weighted residuals (CWRES) in histogram and QQ plot indicated that data met normal distribution.
FIGURE 1.
CWRES (condition weighted residuals) histogram (A) and QQ (quantile–quantile) plot (B).
The stepwise screening process is presented in Table 2. From all 12 covariates, only aspartate aminotransferase (AST) and CYP2C19 genotype were found to exert significant influence on PK parameters and were incorporated into parametric equation. Model 1: V (L) = 22.47 × [1 + 2.21 × (EM = 1)] × [1 + 4.67 × (IM = 1)] × [1 + 3.30 × (PM = 1)] × exp (0.96); CL (L/h) = 4.76 × (AST/33)^(−0.23) × exp (0.14).
TABLE 2.
Results of Stepwise Screening of Individual Covariates
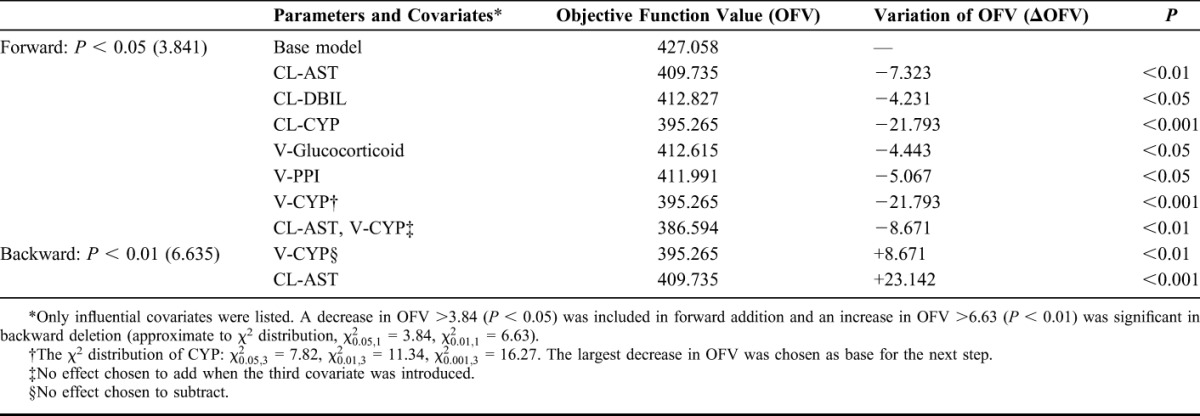
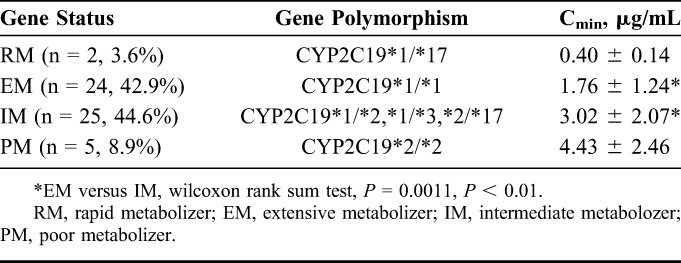
Furthermore, we analyzed the association between VRC dosage form, time after transplant, CYP2C19 genetic polymorphism, and Cmin. RM and PM metabolizers were not statistically analyzed because the sample sizes were only 2 and 5. No significant relationship was found between dosage form, time after transplant, and Cmin. It appeared that Cmin in RMs is significantly lower than in the other 3 genotype subgroups (as shown in Table 3). The differences of VRC Cmin were statistically significant between EMs and IMs (P < 0.05). Moreover, the allele frequencies of CYP2C19*2, CYP2C19*3, and CYP2C19*17 were 53.2%, 3.2%, and 4.8%, respectively.
TABLE 3.
Mean Cmin in Different CYP2C19 Gene Status
Model Evaluation
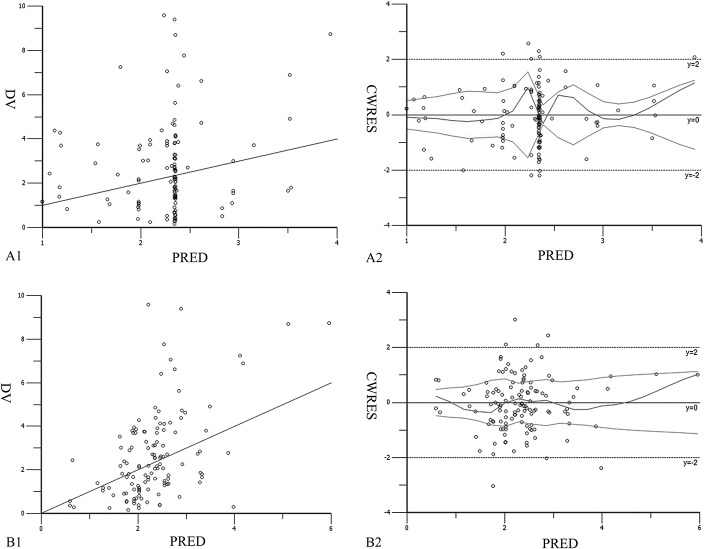
The values of ωV, ωCL, and σ in final model were obviously lower than in the base model, as seen in Table 4. The final model has lower interindividual and residual variation, indicating that CYP2C19 genotype and AST incorporated in equations had a significant effect on VRC pharmacokinetic. The final model allowed more accurate prediction of Cmin. Although the population-predicted concentrations were strongly biased in the base model in scatter plots of detected values versus population-predicted values (Fig. 2A1, B1), the population-predicted concentrations agreed well with the observed voriconazole concentrations in the final model. In addition, CWRES of predicted concentrations of the final model was more uniformly distributed within the range [y = ±2 (±1.96 σ)] (Fig. 2A2, B2). In contrast, the 2 average CWRES trend lines of the base model slightly extended outward at the end. Overall, all of the above illustrates that the final model was more accurate and stable.
TABLE 4.
Comparison of Parameters Between the Basic Model and the Final Model

FIGURE 2.
DV-PRED (detected values vs. population-predicted values) plot and CWRES-PRED (condition weighted residuals vs. population-predicted values) plot of basic model (A1, A2) and final model (B1, B2).
Model Validation—Bootstrap Method
Bootstrap is a recommended internal validation method for PPK model based on data resampling technique. All 1000 bootstraps were completed successfully. The population parameter estimates were similar to the simulation values and fell within 95% CI from bootstrap (Table 5). Hence, the model was confirmed to be stable and accurate.
TABLE 5.
Parameter of the Final Model and Bootstrap Evaluation (1000 Times)

DISCUSSION
Currently, clinicians have increasing interest in prevention and treatment of IFI using VRC. To our knowledge, this is the first PPK study of VRC in RTRs. Recently,17–20 a 1-compartment model was reported to describe the PPK characteristic of VRC in patients. However, there have been conflicting data that support a 2-compartment model with Michaelis clearance.21,22 The inconsistent results may be due to small sample size and limited VRC serum concentration. The V and CL for RTRs were 22.47 L and 4.76 L/h, respectively. By comparison, 2 studies conducted in liver transplant recipients (CL/F = 5.8 ± 5.5 L/h, Vss/F = 94.5 ± 54.9 L, F = 53%–94%) and lung transplant recipients (CL = 3.45 L/h, Vc = 54.7 L, Vp = 143 L) resulted in a similar CL, although different V was observed.23,24 This difference might attribute to the patients' underlying disease and immune status.
As shown in Table 2, AST is an indicator for liver function, which significantly affected CL in this study. A previous clinical trial also demonstrated that PM has a significant decrease on CL.19 CYP2C19 genotype had the same degree of influence on CL and V in stepwise (OFV = 21.793) for this study. Finally, CYP2C19 genotype was incorporated into the equation for V. Also, CL was impacted by direct bilirubin (DBIL) to a certain extent, but DBIL was not identified to be significant in RTRs, although it is a significant factor in patients with pulmonary disease 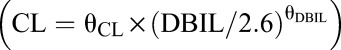 .20 Both PPI and glucocorticoid seem not to be important factors on VRC serum concentration in RTRs. However, it is still under debate, because of different sample size, diversity of PPI, and condition of patients.18,19,21,25,26
.20 Both PPI and glucocorticoid seem not to be important factors on VRC serum concentration in RTRs. However, it is still under debate, because of different sample size, diversity of PPI, and condition of patients.18,19,21,25,26
CYP2C19 genetic polymorphism is a crucial determinant of the extensive pharmacokinetic variability of VRC.21,27 The frequencies of CYP2C19*2 and CYP2C19*3 allele are much higher in Asians (15%–20%) than in Caucasians (2%–3%).28 The allele frequencies of CYP2C19*2, CYP2C19*3, and CYP2C19*17 (53.2%, 3.2%, and 4.8%) in the present study are very similar to those of another study in a Chinese population (50.0%, 6.3%, and 2.1%),27 but it is not consistent with data from other countries (15%, 0.4%, or 0.5%).14,29 Interestingly, one IM (Cmin = 2.18 mcg/mL) is with CYP2C19*2/*17 genotype that rarely appears in other studies. It is probably because the gain-of-function allele CYP2C19*17 is unable to completely compensate for the loss-of-function allele CYP2C19*2.15 Further, the distribution in different populations is distinctly different from various studies,30–33 which showed that Caucasians have a high frequency of *17 allele (49%)34, while other ethnic groups, especially Chinese, have a high frequency of *2 allele (Table 6). The proportion (∼50%) of slow metabolizers in Chinese is far above the average level of other Asian populations (15%–20%),27,29 indicating that Chinese are susceptible to altered pharmacokinetic of VRC due to slow metabolism. Thus, genotyping in advance is necessary for the initial dose.
TABLE 6.
Comparison of Alleles in Different Populations

Although introduction of CYP2C19 genotype and AST into the final model significantly reduces the interindividual variability, the population prediction by the final model showed a deviation at high concentrations, which indicted that incorporated factors only explained a part of the variation of VRC pharmacokinetic in RTRs. One of the reasons is that there is saturated metabolism in patients with loss-of-function mutants.
Population estimates of covariate model were within 95% CI of bootstrap simulation values, which suggested that the model was stable and accurate. At the same time, the wide CI was seen, which indicates that Cmin of VRC should be monitored.
There are several limitations of this study as follows: (1) The sample size is small and only Cmin was analyzed. (2) The results need to be validated in further prospective studies. Nevertheless, our retrospective study provides valuable information for subsequent research on VRC pharmacokinetics/pharmacodynamics (PK/PD). Furthermore, it would be promising to guide individual treatment in RTRs by combining PK/PD based on TDM and genotypes screening.
In conclusion, we have developed PPK model in RTRs: V (L) = 22.47 × [1 + 2.21 × (EM = 1)] × [1 + 4.67 × (IM = 1)] × [1 + 3.30 × (PM = 1)] × exp (0.96); CL (L/h) = 4.76 × (AST/33)^(−0.23) × exp (0.14). Liver function and CYP2C19 polymorphism are major determinants of VRC pharmacokinetic variability in RTRs. VRC Cmin in intermediate metabolizers was significantly higher than in extensive metabolizers. This is the first report about PPK of VRC in RTRs.
ACKNOWLEDGMENTS
The authors thank Lin Li, Zeng-Quan Dong, Ying Li, Ping Yang, and Xiao-Pei Tong for assistance in collection of clinical data and blood samples. They also thank Prof. Hoan Linh Banh from Albert (Canada) and Zhong-Qi Dong for reviewing the final manuscript. In particular, the authors are thankful for the support of clinical project for new technique of Central South University.
Footnotes
Supported by the clinical project for new technique of Central South University.
Z.-W. Li and F.-H. Peng contributed equally to this work. Z.-W. Li and F.-H. Peng participated in the medical management of study patients, data collection, and article preparation and performed the statistical analyses. M. Yan and B.-K. Zhang designed the study protocol and participated in article examination and editing. W. Liang and X.-L. Liu performed the statistical analyses and graphic review. S.-L. Tang, Y.-Q. Wu, and X.-L. Lin participated in DNA extraction and detection. P.-F. Wang participated in blood sampling and concentration analysis. P. Xu, Y.-P. Liu, and P.-F. Fang participated in data extraction and patient chart review. D.-X. Xiang participated in study database management. All authors read and approved the final manuscript.
The authors declare no conflict of interest.
This clinical trial is registered on Chinese Clinical Trial Registry (http://www.chictr.org/en/; Registration number: ChiCTR-RRC-16008314).
REFERENCES
- 1.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50:1101–1111. [DOI] [PubMed] [Google Scholar]
- 2.López-Medrano F, Fernández-Ruiz M, Silva JT, et al. Clinical presentation and determinants of mortality of invasive pulmonary aspergillosis in kidney transplant recipients: a multinational cohort study. Am J Transpl. 2016;16:3220–3234. [DOI] [PubMed] [Google Scholar]
- 3.López-Medrano F, Silva JT, Fernández-Ruiz M, et al. Risk factors associated with early invasive pulmonary aspergillosis in kidney transplant recipients: results from a multinational matched case-control study. Am J Transpl. 2016;16:2148–2157. [DOI] [PubMed] [Google Scholar]
- 4.Heylen L, Maertens J, Naesens M, et al. Invasive aspergillosis after kidney transplant: case-control study. Clin Infect Dis. 2015;60:1505–1511. [DOI] [PubMed] [Google Scholar]
- 5.Desbois AC, Poiree S, Snanoudj R, et al. Prognosis of invasive aspergillosis in kidney transplant recipients: a case-control study. Transpl Direct. 2016;2:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolton MJ, McLachlan AJ. Voriconazole pharmacokinetics and exposure–response relationships: assessing the links between exposure, efficacy and toxicity. Int J Antimicrob Agents. 2014;44:183–193. [DOI] [PubMed] [Google Scholar]
- 9.Dolton MJ, Ray JE, Chen SC, et al. Multicenter study of VRC pharmacokinetics and therapeutic drug monitoring. Antimicrob Agents Chemother. 2012;56:4793–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Racil Z, Winterova J, Kouba M, et al. Monitoring trough voriconazole plasma concentrations in haematological patients: real life multicentre experience. Mycoses. 2012;55:483–492. [DOI] [PubMed] [Google Scholar]
- 11.Hoyo I, Sanclemente G, de la Bellacasa JP, et al. Epidemiology, clinical characteristics, and outcome of invasive aspergillosis in renal transplant patients. Transpl Infect Dis. 2014;16:951–957. [DOI] [PubMed] [Google Scholar]
- 12.Capone D, Tarantino G, Gentile A, et al. Effects of voriconazole on tacrolimus metabolism in a kidney transplant recipient. J Clin Pharm Ther. 2010;35:121–124. [DOI] [PubMed] [Google Scholar]
- 13.Park SJ, Song IS, Kang SW, et al. Pharmacokinetic effect of voriconazole on cyclosporine in the treatment of aspergillosis after renal transplantation. Clin Nephrol. 2012;78:412–418. [DOI] [PubMed] [Google Scholar]
- 14.Mikus G, Scholz IM, Weiss J. Pharmacogenomics of the triazole antifungal agent voriconazole. Pharmacogenomics. 2011;12:861–872. [DOI] [PubMed] [Google Scholar]
- 15.Brad M, Aniwaa OO, Julia B, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC®) Guideline for CYP2C1 and Voriconazole Therapy. 2016. Available at: https://www.pharmgkb.org/guideline/PA166161537. [Google Scholar]
- 16.Guidance for Industry Population Pharmacokinetics. U.S. Department of Health and Human Services Food and Drug Administration. 1999. Available at: http://www.fda.gov/cder/guidance/index.htm. [Google Scholar]
- 17.Farkas A, Daroczi G, Villasurda P, et al. Comparative evaluation of the predictive performance of three different structural population pharmacokinetic models to predict future voriconazole concentrations. Antimicrob Agents Chemother. 2016;60:6806–6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pascual A, Csajka C, Buclin T, et al. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin Infect Dis. 2012;55:381–390. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Chen S, Sun J, et al. Identification of factors influencing the pharmacokinetics of voriconazole and the optimization of dosage regimens based on Monte Carlo simulation in patients with invasive fungal infections. J Antimicrob Chemother. 2014;69:463–470. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Xie H, Liang F, et al. Population pharmacokinetics in China: the dynamics of intravenous voriconazole in critically ill patients with pulmonary disease. Biol Pharm Bull. 2015;38:996–1004. [DOI] [PubMed] [Google Scholar]
- 21.Hope WW. Population pharmacokinetics of voriconazole in adults. Antimicrob Agents Chemother. 2012;56:526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolton MJ, Mikus G, Weiss J, et al. Understanding variability with voriconazole using a population pharmacokinetic approach: implications for optimal dosing. J Antimicrob Chemother. 2014;69:1633–1641. [DOI] [PubMed] [Google Scholar]
- 23.Johnson HJ, Han K, Capitano B, et al. Voriconazole pharmacokinetics in liver transplant recipients. Antimicrob Agents Chemother. 2010;54:852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han K, Capitano B, Bies R, et al. Bioavailability and population pharmacokinetics of voriconazole in lung transplant recipients. Antimicrob Agents Chemother. 2010;54:4424–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoenigl M, Duettmann W, Raggam RB, et al. Potential factors for inadequate voriconazole plasma concentrations in intensive care unit patients and patients with hematological malignancies. Antimicrob Agents Chemother. 2013;57:3262–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Kim BH, Nam WS, et al. Effect of CYP2C19 polymorphism on the pharmacokinetics of voriconazole after single and multiple doses in healthy volunteers. J Clin Pharmacol. 2012;52:195–203. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Zhu H, Sun J, et al. Efficacy and safety of voriconazole and CYP2C19 polymorphism for optimised dosage regimens in patients with invasive fungal infections. Int J Antimicrob Agents. 2014;44:436–442. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu T, Ochiai H, Asell F, et al. Bioinformatics research on interracial difference in drug metabolism I. Analysis on frequencies of mutant alleles and poor metabolizers on CYP2D6 and CYP2C19. Drug Metab Pharmacokinet. 2003;18:48–70. [DOI] [PubMed] [Google Scholar]
- 29.Zonios D, Yamazaki H, Murayama N, et al. Voriconazole metabolism, toxicity, and the effect of cytochrome P450 2C19 genotype. J Infect Dis. 2014;209:1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chawla PK, Nanday SR, Dherai AJ, et al. Correlation of CYP2C19 genotype with plasma voriconazole levels: a preliminary retrospective study in Indians. Int J Clin Pharm. 2015;37:925–930. [DOI] [PubMed] [Google Scholar]
- 31.Kim SH, Lee DG, Kwon JC, et al. Clinical impact of cytochrome P450 2C19 genotype on the treatment of invasive aspergillosis under routine therapeutic drug monitoring of voriconazole in a Korean population. Infect Chemother. 2013;45:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuo LJ, Guo T, Xia DY, et al. Allele and genotype frequencies of CYP3A4, CYP2C19, and CYP2D6 in Han, Uighur, Hui, and Mongolian Chinese populations. Genet Test Mol Biomarkers. 2012;16:102–108. [DOI] [PubMed] [Google Scholar]
- 33.Chuwongwattana S, Jantararoungtong T, Chitasombat MN, et al. A prospective observational study of CYP2C19 polymorphisms and voriconazole plasma level in adult Thai patients with invasive aspergillosis. Drug Metab Pharmacokinet. 2015;31:117–122. [DOI] [PubMed] [Google Scholar]
- 34.Lamoureux F, Duflot T, Woillard JB, et al. Impact of CYP2C19 genetic polymorphisms on voriconazole dosing and exposure in adult patients with invasive fungal infections. Int J Antimicrob Agents. 2016;47:124–131. [DOI] [PubMed] [Google Scholar]