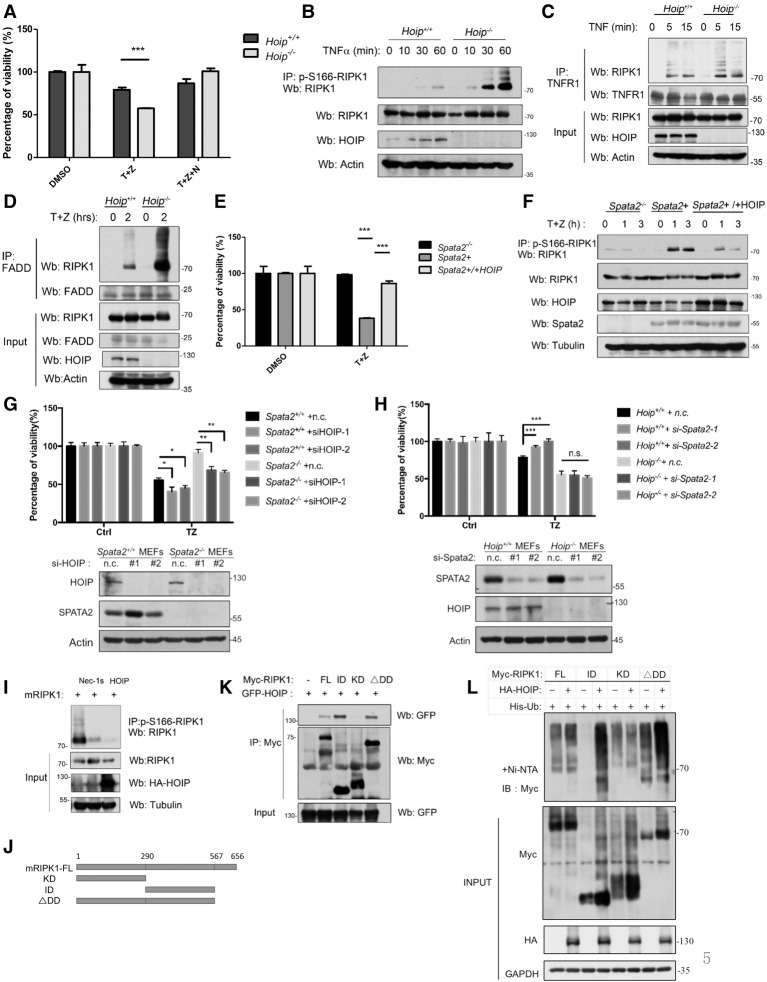
Figure 5.
M1 ubiquitination antagonizes RIPK1 kinase activation. (A) Hoip+/+ MEFs and Hoip−/− MEFs were pretreated with or without 20 µM Nec-1s for 10 min and then treated with 10 ng/mL human TNFα and 20 µM zVAD.fmk for 24 h. Cell viability was measured by CellTiterGlo assay. (B) Hoip+/+ MEFs and Hoip−/− MEFs were treated with 100 ng/mL human TNFα and 50 µM zVAD.fmk for the indicated periods of time. The cell lysates were analyzed by immunoprecipitation with anti-p-S166 RIPK1. The immunocomplexes were analyzed by Western blotting using the indicated antibodies. (C) Hoip+/+ MEFS and Hoip−/− MEFs were treated with 100 ng/mL human TNFα for the indicated periods of time. The cell lysates were subjected to immunoprecipitation using anti-TNFR1 and then analyzed by Western blotting using the indicated antibodies. (D) Hoip+/+ MEFS and Hoip−/− MEFs were treated with 100 ng/mL human TNFα plus 50 µM zVAD.fmk for the indicated periods of time. The cell lysates were subjected to immunoprecipitation using anti-FADD and then analyzed by Western blotting using the indicated antibodies. (E) Spata2−/− MEFs, Spata2+ MEFs, and Spata2+ MEFs overexpressing HOIP were treated with 10 ng/mL human TNFα and 50 µM zVAD.fmk for 24 h. Cell viability was measured by CellTiterGlo assay. (F) Spata2−/− MEFs, Spata2+ MEFs, and Spata2+ MEFs overexpressing HOIP were treated with 10 ng/mL human TNFα and 50 µM zVAD.fmk for the indicated periods of time. The cell lysates were analyzed by immunoprecipitation using anti-p-S166 RIPK1. The immunocomplexes were then analyzed by Western blotting using the indicated antibodies. (G) Spata2+/+ MEFs and Spata2−/− MEFs were transfected with HOIP siRNA or nontargeting control (n.c.) for 24 h and treated with 50 ng/mL TNFα plus 50 µM zVAD.fmk for 16 h. Cell viability and cell death were evaluated using CellTiterGlo assay. (H) Hoip+/+ MEFs and Hoip−/− MEFs were transfected with Spata2 siRNA or nontargeting control (n.c.) for 24 h and treated with 50 ng/mL TNFα plus 50 µM zVAD.fmk for 16 h. Cell viability was evaluated using CellTiterGlo assay. (I) 293T cells were transfected for HA-mRIPK1 with or without HA-HOIP for 24 h and treated with or without 20 µM Nec-1s for the last 6 h. The cell lysates were analyzed by immunoprecipitation using antip-S166 RIPK1. The immunocomplexes were analyzed by Western blotting using the indicated antibodies. (J) Schematic representation of the Myc-tagged mouse RIPK1 truncations used. (K) 293T cells were transfected with expression vectors for different domains of Myc-mRIPK1 (as shown in J) with GFP-HOIP for 24 h. The cell lysates were analyzed by immunoprecipitation using anti-Myc antibody. The immunocomplexes were then analyzed by Western blotting using the indicated antibodies. (L) 293T cells were transfected with expression vectors for different domains of Myc-mRIPK1 with or without HA-HOIP for 24 h. The cell lysates were subjected to His pull-down with Ni-NTA beads and analyzed by Western blotting using the indicated antibodies. Error bars represent SEM from three technical replicates. GAPDH and Tubulin were used as loading controls.