Abstract
Objective
To examine black-white and Hispanic-white differences in total knee arthroplasty from 2001 to 2013 in a large cohort of patients diagnosed with osteoarthritis in the Veterans Affairs (VA) Healthcare System.
Methods
Data were from the VA Musculoskeletal Disorders Cohort, which includes data from electronic health records of over 5.4 million veterans with musculoskeletal disorders diagnoses. We included white (non-Hispanic), black (non-Hispanic), and Hispanic (any race) veterans age 50 years or older with an osteoarthritis diagnosis from 2001-2011 (N=539,841). Veterans were followed from their first osteoarthritis diagnosis until September 30, 2013. As a proxy for increased clinical severity, analyses were also conducted for a sub-sample restricted to those who saw an orthopedic or rheumatology specialist (N=148,844). We used Cox proportional hazards regression to examine racial and ethnic differences in total knee arthroplasty by year of osteoarthritis diagnosis, adjusting for age, sex, body mass index, physical and mental diagnoses, and pain intensity scores.
Results
We identified 12,087 total knee arthroplasty procedures in a sample of 473,170 white, 50,172 black, and 16,499 Hispanic veterans. In adjusted models examining black-white and Hispanic-white differences by year of OA diagnosis, total knee arthroplasty rates were lower for black than for white veterans diagnosed in all but two years. There were no Hispanic-white differences regardless of when diagnosis occurred. These patterns held in the specialty clinic sub-sample.
Conclusions
Black-white differences in total knee arthroplasty appear to be persistent in the VA, even after controlling for potential clinical confounders.
Knee osteoarthritis (OA) is one of the most common forms of arthritis and a significant source of disability.(1, 2) Total knee arthroplasty (TKA) is a well-established surgical treatment for advanced cases that can no longer be managed using more conservative treatments.(3) Despite the availability of this effective procedure, studies have documented lower rates of TKA among people of non-Hispanic black/African American race or Hispanic ethnicity than among non-Hispanic whites.(4-10) Studies of TKA rates over time show that, although overall use of TKA has increased across most racial and ethnic subgroups, rates among non-Hispanic black and Hispanic subgroups remain lower than among non-Hispanic whites.(6, 10-12)
Prior studies examining time trends in racial and ethnic differences in TKA have relied on cross-sectional comparisons of TKA procedure rates across racial and ethnic groups in the general population at different points in time.(6, 10-12) Cross-sectional studies of the general population do not take into account the slow, degenerative nature of OA and possible clinical and demographic differences across racial and ethnic groups that may impact clinical need for the procedure. Most prior studies have also relied on publicly available data from non-federal hospitals, thereby excluding patients served by the largest integrated healthcare system in the United States, the Veterans Affairs (VA) Healthcare System.(5, 6, 10, 12, 13)
To address these gaps in the literature, we identified a large cohort of veterans who were diagnosed with OA while receiving care in the VA from 2001-2011. We followed this cohort through September 30, 2013 to determine if racial or ethnic differences in receipt of TKA have varied over time. We focused specifically on black-white and Hispanic-white differences, as blacks and Hispanics are the two largest minority groups in the VA patient population and Hispanic-white differences in TKA have not previously been examined in the VA. Our study further improves upon prior studies by adjusting for demographic and clinical factors (e.g., age, sex, pain intensity ratings, comorbid conditions) that may impact appropriateness of TKA and account for racial and ethnic differences in TKA. To focus the analysis on those who are most likely to be appropriate candidates for TKA, we also conducted sensitivity analyses restricted to a specialty clinic sub-sample of patients who had visited either an orthopedist or rheumatologist during the observation period, as patients with more advanced OA are often seen by these specialists prior to undergoing TKA.
Methods
Data for this study were from the VA Musculoskeletal Disorders (MSD) Cohort, a comprehensive registry of all veterans with MSD diagnoses who received care in a VA inpatient and/or outpatient medical facility between 2000 and 2013.(14) To be eligible for the MSD Cohort, a veteran had to have an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9CM) code consistent with 1 of 1,685 distinct MSD diagnoses. The MSD index date was determined by the date of the first inpatient MSD code or the date of an initial outpatient code in the observation period for which there is a second confirmatory outpatient code in the following 18 months. Additional demographic and clinical information was extracted from VA electronic clinical and administrative data sources in the VA Corporate Data Warehouse for all eligible veterans both prior to and following the date of their first MSD diagnosis to enable the longitudinal analysis of numerous pain treatments and outcomes. The VA Connecticut Healthcare System Institutional Review Board approved the MSD Cohort and the analyses reported here prior to data collection.
For the current analysis, we focused on veterans who entered the MSD Cohort with OA between 2001 and 2011. We focused on these years because we were interested in following veterans with new OA diagnoses (by excluding veterans who entered the MSD cohort in 2000) and to allow at least a 2-year follow up period (by excluding veterans who entered in 2012 or 2013). We included veterans with any OA diagnosis (ICD-9CM=715.xx) recorded at ≥2 outpatient visits within 18 months of one another or ≥1 inpatient stay in the observation period. The date of the first OA diagnosis in the years 2001 to 2011 was used as the index date. We excluded those who had TKA in one or both knees on or before their first OA diagnosis within the study timeframe. Because we were interested in TKA utilization as an elective treatment for OA, we also excluded veterans who had a diagnosis of rheumatoid arthritis (ICD-9 714.xx) or were less than 50 years old, as TKA is often not recommended for younger patients given the longevity of the prosthesis. We further excluded veterans who had a racial/ethnic classification other than non-Hispanic white, non-Hispanic black, or Hispanic or were missing racial/ethnic information. Veterans who met these criteria were followed from their OA index date until receipt of TKA, death (from the VA Beneficiary Identification Records Locator Subsystem), or September 30, 2013, whichever came first.
Veterans who met the previous criteria were included in the main analyses, with each veteran being counted only one time and tracked from their OA index date. We also conducted subgroup analyses in which we further restricted the analytic sample to veterans who met all eligibility criteria and had one or more visits to either an orthopedist or rheumatologist (VA clinic codes 409 and 314, respectively) after their first OA diagnosis but on or prior to the date of TKA. The purpose of this analysis was to determine whether racial and ethnic differences in TKA occur among patients with more advanced OA, as patients with OA are typically referred to an orthopedist or rheumatologist once their OA has progressed to the point when it can no longer be treated adequately in primary care. The specialty clinic subgroup analysis also would indicate whether racial or ethnic differences observed in the full OA sample could be due to differential referral to or use of specialists.
Primary Predictor: Race/Ethnicity
In the VA, self-identified race and ethnicity is collected from veterans at the time they enroll for services and may be updated at every clinical encounter. Veterans are permitted to specify multiple races as well as indicate whether they are of Hispanic or Latino ethnicity. For this study, we used the latest available non-missing race and ethnicity recorded in each veteran's VA administrative record. We filled in missing values with the most recent race and ethnicity data from the Centers for Medicare and Medicaid Services Master Beneficiary Summary File whenever possible, which resulted in relatively few veterans being excluded due to missing race/ethnicity (n=10,163, 1.6%). We categorized veterans with non-missing racial/ethnic information into three mutually-exclusive groups for analyses: non-Hispanic white (i.e., whites), non-Hispanic black (i.e., black), or Hispanic ethnicity. In accordance with the latest standards for classification of federal data on race and ethnicity (15), veterans of Hispanic ethnicity were categorized as Hispanic regardless of whether they were also identified as white, black, or another race.
Covariates
Covariates included age, sex, and pain intensity scores on the OA index date. Pain intensity was assessed using the pain intensity numeric rating scale (NRS) score from the VA Vital Signs data.(16, 17) The NRS is used in routine clinical care to screen for pain by asking the patient, “On a scale of 0 to 10, where 0 means no pain and 10 means the worst possible pain, what is your current pain level?” Using the highest NRS score recorded on the day of OA diagnosis, we categorized pain intensity into four levels: 0, no pain; 1 to 3, mild; 4 to 6, moderate; and 7 or greater, severe.(18, 19) Body mass index (BMI) was calculated from the height and weight recorded on the OA index date or, if not recorded on the index date, based on those measures recorded within the prior 12 months or up to six months after.
We also identified comorbid diagnoses concurrent with the OA index diagnosis. Specifically, concurrent conditions were those that occurred within the prior 12 months or up to six months after the index OA diagnosis; these conditions also required 2 outpatient codes within 18 months of one another or 1 inpatient code. We controlled for overall clinical severity by calculating the Charlson Comorbidity Index (CCI), with higher scores indicating greater comorbidity.(20, 21) We also included four mental health diagnoses that are not included in the CCI: depressive disorder, alcohol use disorder, drug use disorder, and post-traumatic stress disorder (PTSD) (see eTable 1 for ICD-9CM codes). We controlled for these conditions because they have high prevalence among veterans, may vary across race and ethnicity, and may be viewed as contraindications for TKA.(22, 23)
Outcome: Receipt of TKA
Our study outcome was receipt of TKA within a VA facility, defined using Current Procedural Terminology (CPT) and ICD-9CM procedure codes (CPT 27447 or ICD-9CM 81.54).
Statistical Analyses
Descriptive statistics for all clinical and demographic characteristics by racial/ethnic category were compared using Analyses of Variance (ANOVA) for continuous variables and a χ2 test or Fisher exact test for categorical variables. We used Cox proportional hazards regression to examine racial and ethnic differences in the rate of TKA by the year of OA diagnosis, after adjusting for age, sex, CCI, depression, alcohol use disorder, drug use disorder, PTSD, BMI, and pain intensity. We conducted all analyses in the full analytic sample and in the specialty clinic sub-sample. Veterans who died or did not have TKA by September 30, 2013 were censored in these analyses.
Because excluding cases with missing values may bias results,(24) missing values on BMI (n=15,552, 2.9%) and pain intensity NRS scores (n=63,243, 11.7%) were imputed by multiple imputation using the SAS MI procedure (SAS 9.4, Cary, NC). The imputation model included race/ethnicity, age at OA diagnosis, year of OA diagnosis, CCI, and whether or not the patient received TKA. Five datasets were imputed and the PROC MIANALYSE procedure in SAS was used to combine the hazard ratio estimates according to Rubin's rules.(25) We conducted a sensitivity analysis comparing the results of a complete case analysis with the results using the imputed data and found that results were substantively the same. We chose to report results from multivariable models based on imputed data. All analyses were conducted using SAS 9.4 (SAS, Cary, NC).
Role of funding source
This work was funded by the Veterans Health Administration. The funding source had no involvement in the design or conduct of the study.
Results
Sample Characteristics
A total of 539,841 veterans (10.0%) in the MSD Cohort met the inclusion criteria for this analysis (Figure 1). Of those, 148,844 (27.6%) had a visit with an orthopedic surgeon or rheumatologist and were included in sensitivity analyses restricted to the specialty clinic sub-sample. For the racial and ethnic comparisons, 473,170 (87.6%) veterans were categorized as non-Hispanic white, 50,172 (9.3%) as non-Hispanic black, and 16,499 (3.1%) as Hispanic. Among those included in the Hispanic category, 13% had no other racial or ethnic classification on file, 78% had a white race classification on file, and 5% had a black race classification on file. There were significant differences in all demographic and clinical characteristics across the three racial and ethnic groups (Table 1). Compared to white veterans, black and Hispanic veterans were younger; more likely to have CCI scores ≥3; and more likely to have depression, PTSD, and alcohol use diagnoses. Compared to whites or Hispanics, blacks were more likely to be female, have drug use diagnoses, and report severe pain intensity, and were less likely to be overweight or obese (defined as BMI >25). Similar racial and ethnic differences were observed within the specialty clinic sub-sample, although this group included higher percentages of veterans who were black or Hispanic, younger, female, obese, and had more mental health diagnoses than the full sample (Table 1). Pain intensity ratings were also higher in the specialty clinic sub-sample than in the full analytic sample (Table 1).
Figure 1.
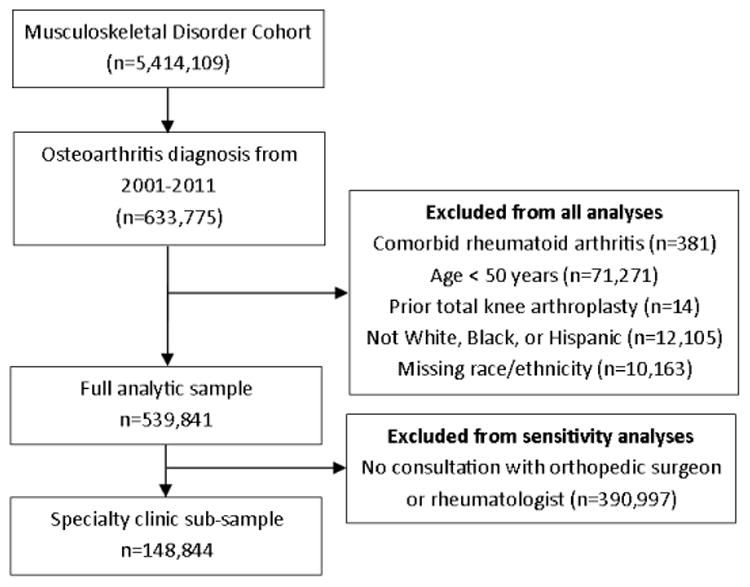
Identification of the Full and Sub-group Analytic Samples. The analyses included veterans who entered the Musculoskeletal Disorders Cohort between 2001 and 2011 with a qualifying diagnosis of osteoarthritis. Veterans were excluded if they had rheumatoid arthritis, were less than 50 years old, had already had total knee arthroplasty, or who had a racial/ethnic classification other than non-Hispanic white, non-Hispanic black, or Hispanic or were missing racial/ethnic information. We conducted sensitivity analyses in a subsample restricted to those who had one or more visits to an orthopedist or rheumatologist.
Table 1.
Characteristics of Veterans age 50 years or older with a diagnosis of osteoarthritis from 2001-2011, by race and ethnicity.
| Characteristic** | Full analytic sample (n=539,841) | Specialty clinic sub-sample (n=148,844)* | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| White | Black | Hispanic | White | Black | Hispanic | |
| n=473,170 | n=50,172 | n=16,499 | n=123,956 | n=18,865 | n=6,023 | |
| (87.6%) | (9.3%) | (3.1%) | (83.3%) | (12.7%) | (4.0%) | |
| Age, % | ||||||
| 50-54 | 7.2 | 20.5 | 12.7 | 13.6 | 27.7 | 18.7 |
| 55-59 | 12.6 | 20.8 | 17.5 | 21.1 | 25.2 | 23.4 |
| 60-64 | 16.1 | 16.3 | 16.8 | 21.4 | 17.1 | 19.0 |
| 65-69 | 13.5 | 10.8 | 12.4 | 12.6 | 9.7 | 11.7 |
| 70-74 | 15.5 | 10.5 | 13.4 | 11.3 | 8.6 | 10.5 |
| ≥75 | 35.1 | 21.1 | 27.3 | 20.0 | 11.8 | 16.8 |
| Female, % | 2.2 | 3.1 | 1.5 | 2.8 | 3.9 | 2.1 |
| CCI, n % | ||||||
| 0 | 55.1 | 53.6 | 52.7 | 58.5 | 58.9 | 55.0 |
| 1-2 | 36.6 | 35.2 | 36.8 | 34.1 | 32.2 | 36.0 |
| ≥3 | 8.3 | 11.2 | 10.5 | 7.5 | 8.9 | 8.9 |
| Depressive disorder, % | 2.3 | 3.2 | 4.2 | 3.7 | 3.9 | 5.4 |
| Alcohol use disorder, % | 3.5 | 9.2 | 5.7 | 5.1 | 9.8 | 7.0 |
| Drug use disorder, % | 0.8 | 6.1 | 1.7 | 1.4 | 7.2 | 2.5 |
| PTSD, % | 4.6 | 8.9 | 8.5 | 7.5 | 10.4 | 12.1 |
| BMI, % | ||||||
| <18.5 | 0.7 | 1.7 | 0.9 | 0.4 | 0.9 | 0.3 |
| 18.5-24.9 | 17.4 | 21.3 | 18.1 | 13.7 | 17.0 | 13.0 |
| 25.0-29.9 | 39.0 | 33.7 | 39.8 | 35.9 | 32.8 | 38.3 |
| ≥30.0 | 40.0 | 40.5 | 39.5 | 47.7 | 46.6 | 46.7 |
| Missing | 2.9 | 2.8 | 1.7 | 2.3 | 2.6 | 1.7 |
| Pain intensity, % | ||||||
| No pain | 51.3 | 41.0 | 45.6 | 36.5 | 30.9 | 35.1 |
| Mild | 12.5 | 9.4 | 13.5 | 13.6 | 9.2 | 11.8 |
| Moderate | 15.1 | 16.7 | 14.2 | 20.1 | 18.9 | 17.5 |
| Severe | 9.6 | 19.0 | 13.4 | 14.9 | 24.3 | 18.9 |
| Missing | 11.4 | 13.9 | 13.4 | 15.0 | 16.8 | 16.6 |
| Days to TKA, Median | 1303 | 1465 | 1408 | 1224 | 1408 | 1326 |
| Years Follow Up, Median | 8 | 7 | 8 | 8 | 8 | 8 |
BMI=body mass index; CCI=Charlson Comorbidity Index; PTSD=post-traumatic stress disorder; TKA=total knee arthroplasty
Restricted to those in the full analytic sample who also consulted with an orthopedic surgeon or rheumatologist within the study timeframe.
All omnibus tests comparing white, black, and Hispanic groups were significant at p<0.001 in the full analytic sample and in the specialty clinic sub-sample.
Predictors of TKA in the Full Analytic Sample
We identified 12,087 TKA procedures during a median follow-up period of 8 years, for an overall rate of 2.2%. In a model examining racial and ethnic differences by year of OA diagnosis and adjusting for age, sex, comorbidity, BMI, and pain intensity, blacks were less likely than whites to undergo TKA for those diagnosed in all but two years of the observation period (see Figure 2 for year-specific black-white and Hispanic-white hazard ratios; see eTable 2 for full model results). Likelihood of undergoing TKA was not significantly different between Hispanics and whites for those diagnosed in any of the years of observation (Figure 2; eTable 2). As shown in Figure 3, likelihood of undergoing TKA decreased as age and CCI scores increased and was significantly lower among those with PTSD. In contrast, likelihood of undergoing TKA increased as BMI and pain intensity increased.
Figure 2.
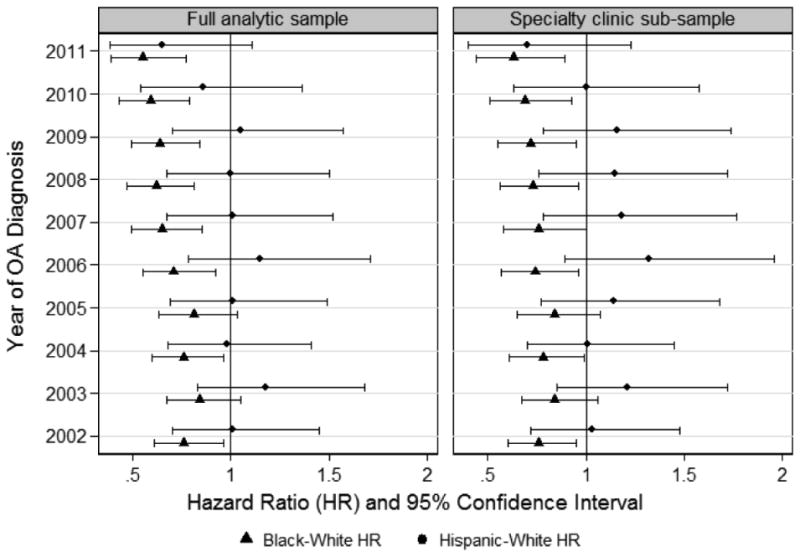
Racial and Ethnic Differences in Total Knee Arthroplasty by Year of Osteoarthritis Diagnosis.
Black-white and Hispanic-white hazard ratios for undergoing total knee arthroplasty by September 30, 2013, plotted by year of osteoarthritis diagnosis. Specialty clinic sub-sample included those who consulted with an orthopedic surgeon or rheumatologist within the study timeframe. Models adjusted for age, sex, Charlson Comorbidity Index, body mass index, pain severity, and diagnoses of depression, alcohol use disorder, drug use disorder, and post-traumatic stress disorder.
Figure 3.
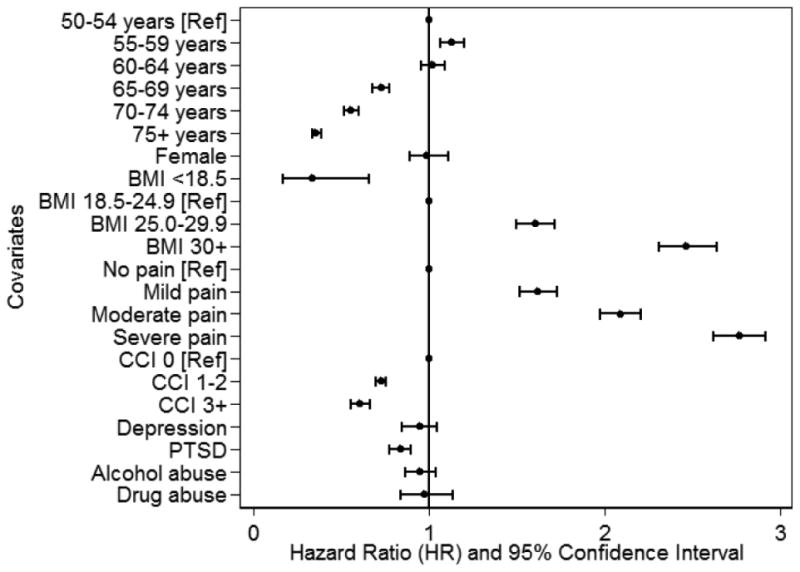
Predictors of Total Knee Arthroplasty Other than Race/Ethnicity.
Estimates are from analyses conducted on the full analytic sample. Models included all covariates plus indicators for black race, Hispanic ethnicity, year of osteoarthritis diagnosis, and all race/ethnicity by year interactions (see eTable 2 for full model results).
Racial and Ethnic Differences in TKA in the Specialty Clinic Sub-sample
In analyses restricted to the specialty clinic sub-sample, there were 12,025 TKA procedures performed, for an overall rate of 8.1%. Patterns of racial and ethnic differences in the adjusted models were similar to those observed in the full analytic sample, in that blacks were less likely than whites to undergo TKA for those diagnosed in all but two years of the observation period and there were no Hispanic-white differences among those diagnosed in any years (Figure 2 and eTable 2).
Discussion
In this longitudinal analysis of a large national cohort of veterans, we found lower likelihood of TKA for blacks than for whites diagnosed with OA in most years from 2001 to 2011. In contrast, we found no difference in the likelihood of TKA between Hispanics and whites diagnosed in any year. These patterns were observed after controlling for racial and ethnic differences in demographic and clinical characteristics that could confound the association between race/ethnicity and receipt of TKA, including age, sex, physical and mental comorbidities, BMI, and pain intensity. The TKA patterns also persisted among the sub-sample of veterans who had consulted with an orthopedic surgeon or rheumatologist. The consistent pattern of black-white differences in receipt of TKA in the overall sample of veterans with OA and in the specialty clinic sub-sample suggest that the observed racial differences were not due to differences in clinical severity, as most patients with OA are not seen in these specialty clinics until their disease has advanced beyond what can be adequately managed in primary care. It is important to note that, not only were pain intensity ratings higher across all racial and ethnic groups in the specialty clinic sub-sample, the percentages of black and Hispanic veterans in the sub-sample were also slightly higher than in the overall sample with OA. The latter finding suggests that systematic differences in referral to, or use of, these specialists are unlikely to explain the black-white differences in TKA we observed in this VA cohort.
Our study builds upon previous work documenting lower rates of TKA in black veterans with OA compared to their white counterparts.(26) Not only do we replicate previous findings in a more comprehensive national cohort of veterans over a longer time frame, we demonstrate that the black-white difference in receipt of TKA has persisted in recent years. The black-white differences in TKA observed among veterans is similar to patterns found in studies showing that racial differences in TKA have persisted or increased over time outside the VA Healthcare System.(6, 10, 11) However, the VA is a unique setting in which to examine potential disparities because it is an integrated healthcare system where all patients must meet certain eligibility requirements to gain access and, once enrolled, have similar access to services. Our findings therefore provide strong support for the conclusion that lower utilization of TKA among blacks cannot be fully explained by differences in healthcare access.
Identifying why black-white differences in TKA persist in the VA remains a challenge. The differences are not likely due to differences in clinical need, as all patients included in the analyses had a diagnosis of OA. Additionally, we controlled for pain intensity at the time of diagnosis, which was higher among blacks and was an independent predictor of receipt of TKA. Differences in access to the procedure likely do not explain our finding, as all patients were receiving care in the VA Healthcare System. However, we might consider whether black veterans are disproportionately served in VA facilities with lower volumes of TKA procedures, overall. Another possible factor that has not been examined is whether there are racial differences in the opportunity costs of having surgery (e.g., time off work, etc.) or the availability of social support during the recovery period.
There is considerable evidence that much of the black-white difference in receipt of TKA is driven by differences in patient preferences for arthritis treatments.(27-30) Compared to whites, blacks are less willing to consider joint replacement as a treatment option for arthritis, and are more willing to seek out non-invasive treatment or coping strategies such as physical therapy, massage therapy, or prayer.(29, 31, 32) While we were unable to ascertain treatment preferences using the data available for this study, our findings of persistently lower rates of TKA among black veterans with OA are consistent with this explanation.
This is the first study to examine Hispanic-white differences in TKA within the VA. In contrast to some studies in non-veteran populations,(4, 6, 11) we observed no Hispanic-white differences in receipt of TKA among veterans. One possible reason that we did not find significant ethnic differences could be the relatively low number of Hispanics in our cohort. Due to citizenship and education requirements for military service, it is also possible that there are fewer differences between Hispanic and non-Hispanic white veterans than between Hispanic and non-Hispanic white patients in the civilian population. Future work is needed to determine whether differences between Hispanic veterans and civilians in characteristics such as acculturation, language, and socioeconomic status account for smaller Hispanic-white differences across VA and non-VA healthcare settings.
This study contributes to a large literature on racial and ethnic differences in the prevalence, impact, and treatment of OA, with a specific focus on long-standing differences in receipt of TKA.(33-38) In the 3-generation framework of health disparities research,(39) this is a first generation study that documents a persistent black-white difference in receipt of TKA among veterans. An abundance of second-generation studies have attempted to determine factors that explain lower use of TKA among black patients (e.g., patient preferences) within and outside the VA setting,(27, 29, 30, 33, 36, 37, 40-44) and a handful of studies have tested interventions to reduce the difference in receipt of TKA, with limited success.(45-48) Our study underscores the need to continue monitoring and working to reduce racial and ethnic differences in TKA, as black-white differences persist despite targeted efforts to reduce racial differences in this procedure.
Given the persistence of black-white differences in TKA, it may be appropriate to take steps to ensure the pain management needs of black veterans with OA are being met, independent of whether patients receive TKA. For example, we observed higher pain intensity ratings among black patients. To meet the needs of all veterans with OA, it may be necessary to pursue less invasive and/or complementary pain management strategies that preserve function for as long as possible, while continuing to develop interventions reduce black-white differences in receipt of TKA.
Limitations
Conclusions based on this study should be considered in light of limitations that are inherent in the VA administrative data upon which it was based. Our use of race and ethnicity documented in administrative records may have resulted in some misclassification of veterans into the three racial and ethnic groups compared in this study. We were also unable to examine TKA separately for subgroups of Hispanic veterans who had an additional minority race classification on file due to the small number of people in those groups. Because eligibility was based on diagnoses of general OA in VA electronic health records, our cohort is an approximation of the true population of Veterans with knee OA, and our OA index date may not represent the initial onset of OA for all veterans in the analysis. We used all OA diagnostic codes, rather than restricting diagnoses to those indicative of knee OA, because the specific joint affected by OA is often not reflected in the diagnostic code applied to a visit. This is particularly problematic for knee OA because the ICD-9CM system that was in effect during the study timeframe did not include a specific code for knee OA. Under the ICD-9CM system, diagnoses of knee OA could be recorded using general OA codes or conditions specific to regions close to the knee (e.g., lower extremity, hip, foot/ankle). Given the uncertainty regarding how specific ICD-9CM codes were applied to cases of knee OA, we chose to include in our cohort patients with any OA diagnoses. This decision may have resulted in patients with OA in other regions of the body being included in our analyses.
While it would have been ideal to use the strict criteria for knee OA established by American College of Rheumatology to determine specific diagnoses or disease severity based on radiological assessment, this was not feasible given the size of the VA patient population or the data available. Because we did not have access to non-VA medical records, we also could not determine whether veterans in our analysis were being treated for OA outside the VA prior to or after receiving a diagnosis of OA within the VA. Although we attempted to approximate severity of OA using two variables that were available to us (i.e., pain intensity ratings and being seen by an orthopedist or rheumatologist), we recognize that both variables are limited in that we were unable to determine whether they were directly linked to OA, as opposed to other conditions. Despite these limitations, this work fills important gaps in knowledge regarding changes in racial and ethnic differences in TKA use among veterans over time.
Conclusions
In the VA Healthcare System, black veterans diagnosed with OA in most years from 2001 to 2011 were less likely to undergo TKA than white veterans by 2013 after adjusting for demographic and key clinical characteristics. In contrast, Hispanics and whites received TKA at similar rates across all years. To ensure that the health care needs of all veterans are being met, it is important to continue efforts to understand and develop interventions to reduce racial differences in TKA in veterans.
Supplementary Material
eTable 1: International Classification of Diseases, 9th Revision, Clinical Modification (ICD9-CM) codes used to determine mental health diagnoses included as covariates
eTable 2: Hazard ratios of all predictors included in adjusted models for full analytic sample and specialty clinic sub-sample
Significance and Innovations.
-
-
To date, this is the largest study of racial and ethnic differences in total knee arthroplasty in the Veterans Affairs (VA) Healthcare System, including all veterans diagnosed with osteoarthritis in the VA from 2001 to 2011.
-
-
This is the first study to examine Hispanic-white differences in total knee arthroplasty within the VA.
-
-
The large study population, long follow-up period, and availability of demographic and clinical information in VA electronic health records make it possible to examine whether racial and ethnic differences in total knee arthroplasty vary across year of initial osteoarthritis diagnosis after controlling for confounders such as age and pain intensity.
Acknowledgments
The authors thank Melissa Skanderson from the Musculoskeletal Diagnosis Cohort Study for her assistance with preparing the data for this analysis. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, Department of Veterans Affairs, or the United States Government.
This work was supported by the Veterans Health Administration Health Services Research & Development (CREATE 12-012, Principal Investigators: Goulet and Brandt). The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, Department of Veterans Affairs, or the United States Government.
Footnotes
Financial Support/Disclosures: The authors have no conflict of interests to report.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60(12):3546–53. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–62. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among medicare patients. N Engl J Med. 2003;349:1350–9. doi: 10.1056/NEJMsa021569. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Rizzo JA, Parasuraman S, Gunnarsson C. Racial disparities in receiving total hip/knee replacement surgery: the effect of hospital admission sources. J Health Care Poor Underserved. 2013;24(1):135–51. doi: 10.1353/hpu.2013.0026. [DOI] [PubMed] [Google Scholar]
- 6.Bang H, Chiu YL, Memtsoudis SG, Mandl LA, Della Valle AG, Mushlin AI, et al. Total hip and total knee arthroplasties: trends and disparities revisited. Am J Orthop. 2010;39(9):E95–102. [PubMed] [Google Scholar]
- 7.Dunlop DD, Manheim LM, Song J, Sohn MW, Feinglass JM, Chang HJ, et al. Age and racial/ethnic disparities in arthritis-related hip and knee surgeries. Med Care. 2008;46(2):200–8. doi: 10.1097/MLR.0b013e31815cecd8. [DOI] [PubMed] [Google Scholar]
- 8.Dunlop DD, Song J, Manheim LM, Chang RW. Racial disparities in joint replacement use among older adults. Med Care. 2003;41(2):288–98. doi: 10.1097/01.MLR.0000044908.25275.E1. [DOI] [PubMed] [Google Scholar]
- 9.Hanchate AD, Zhang Y, Felson DT, Ash AS. Exploring the determinants of racial and ethnic disparities in total knee arthroplasty: health insurance, income, and assets. Med Care. 2008;46(5):481–8. doi: 10.1097/MLR.0b013e3181621e9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh JA, Lu X, Rosenthal GE, Ibrahim S, Cram P. Racial disparities in knee and hip total joint arthroplasty: an 18-year analysis of national Medicare data. Ann Rheum Dis. 2014;73(12):2107–15. doi: 10.1136/annrheumdis-2013-203494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurer A, Jones LC. Musculoskeletal healthcare disparities: influence of patient sex, race, and ethnicity on utilization of total joint arthroplasty. J Long Term Eff Med Implants. 2014;24(2-3):233–40. doi: 10.1615/jlongtermeffmedimplants.2014010610. [DOI] [PubMed] [Google Scholar]
- 12.Cisternas M, Murphy L, Croft J, Helmick C. Racial disparities in total knee replacement among medicare enrollees-United States, 2000-2006. MMWR Morb Mortal Wkly Rep. 2009;58(6):133–8. [PubMed] [Google Scholar]
- 13.Singh JA, Lewallen D. Age, gender, obesity, and depression are associated with patient-related pain and function outcome after revision total hip arthroplasty. Clin Rheumatol. 2009;28(12):1419–30. doi: 10.1007/s10067-009-1267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulet JL, Kerns RD, Bair M, Becker WC, Brennan P, Burgess DJ, et al. The Muskuloskeletal Diagnosis Cohort: examining pain and pain care among veterans. PAIN. 2016;157(8):1696–703. doi: 10.1097/j.pain.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Revisions to the standards for the classification of federal data on race and ethnicity. Fed Regist. 1997;62:58781–90. [Google Scholar]
- 16.Dobscha SK, Morasco BJ, Kovas AE, Peters DM, Hart K, McFarland BH. Short-term variability in outpatient pain intensity scores in a national sample of older veterans with chronic pain. Pain Med. 2015;16(5):855–65. doi: 10.1111/pme.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strong J, Ashton R, Chant D. Pain intensity measurement in chronic low back pain. Clin J Pain. 1991;7(3):209–18. doi: 10.1097/00002508-199109000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Goulet J, Brandt C, Crystal S, Fiellin D, Gilbert C, Gordon A, et al. Agreement between electronic medical record-based and self-administered pain numeric rating scale: clinical and research implications. Med Care. 2013;51:245–50. doi: 10.1097/MLR.0b013e318277f1ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan G, Jensen MP, Thornby JI, Rintala DH, Anderson KO. Categorizing pain in patients seen in a veterans health administration hospital: Pain as the fifth vital sign. Psychological Services. 2008;5(3):239–50. [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 21.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 22.Tierney WM, Fitzgerald JF, Heck DA, Kennedy JM, Katz BP, Melfi CA, et al. Tricompartmental knee replacement. A comparison of orthopaedic surgeons' self reported performance rates with surgical indications, contraindications, and expected outcomes. Knee Replacement Patient Outcomes Research Team Clin Orthop Relat Res. 1994;(305):209–17. [PubMed] [Google Scholar]
- 23.Wright JG, Coyte P, Hawker G, Bombardier C, Cooke D, Heck D, et al. Variation in orthopedic surgeons' perceptions of the indications for and outcomes of knee replacement. CMAJ: Canadian Medical Association Journal. 1995;152(5):687–97. [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, New Jersey: John Wiley & Sons; 2004. [Google Scholar]
- 26.Jones A, Kwoh CK, Kelley ME, Ibrahim SA. Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis Rheum. 2005;53(6):979–81. doi: 10.1002/art.21596. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Differences in expectation of outcome mediate African-American/White patient differences in “willingness” to consider joint replacement. Arthritis Rheum. 2002;46:2429–35. doi: 10.1002/art.10494. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Understanding ethnic differences in the utilization of joint replacement for osteoarthritis: the role of patient-level factors. Med Care. 2002;40(1 Suppl):I44–I51. doi: 10.1097/00005650-200201001-00006. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Variation in perceptions of treatment and self-care practices in elderly with osteoarthritis: a comparison between African American and white patients. Arthritis Rheum. 2001;45(4):340–5. doi: 10.1002/1529-0131(200108)45:4<340::AID-ART346>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Hausmann LRM, Mor M, Hanusa BH, Zickmund S, Cohen PZ, Grant R, et al. The effect of patient race on total joint replacement recommendations and utilization in the orthopedic setting. J Gen Intern Med. 2010;25(9):982–8. doi: 10.1007/s11606-010-1399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones AC, Kwoh CK, Groeneveld PW, Mor M, Geng M, Ibrahim SA. Investigating racial differences in coping with chronic osteoarthritis pain. J Cross Cult Gerontol. 2008;23(4):339–47. doi: 10.1007/s10823-008-9071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figaro MK, Russo PW, Allegrante JP. Preferences for arthritis care among urban African Americans: “I don't want to be cut”. Health Psychol. 2004;23(3):324–9. doi: 10.1037/0278-6133.23.3.324. [DOI] [PubMed] [Google Scholar]
- 33.Ibrahim SA. Racial variations in the utilization of knee and hip joint replacement: an introduction and review of the most recent literature. Curr Orthop Pract. 2010;21(2):126–31. doi: 10.1097/BCO.0b013e3181d08223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irgit K, Nelson CL. Defining racial and ethnic disparities in THA and TKA. Clin Orthop Relat Res. 2011;469(7):1817–23. doi: 10.1007/s11999-011-1885-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolen J, Schieb L, Hootman JM, Helmick CG, Theis K, Murphy LB, et al. Differences in the prevalence and severity of arthritis among racial/ethnic groups in the United States, National Health Interview Survey, 2002, 2003, and 2006. Prev Chronic Dis. 2010;7(3):A64. [PMC free article] [PubMed] [Google Scholar]
- 36.Allen KD, Oddone EZ, Coffman CJ, Keefe FJ, Lindquist JH, Bosworth HB. Racial differences in osteoarthritis pain and function: potential explanatory factors. Osteoarthritis Cartilage. 2010;18(2):160–7. doi: 10.1016/j.joca.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Groeneveld PW, Kwoh CK, Mor MK, Appelt CJ, Geng M, Gutierrez JC, et al. Racial differences in expectations of joint replacement surgery outcomes. Arthritis Rheum. 2008;59(5):730–7. doi: 10.1002/art.23565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ang DC, Ibrahim SA, Burant CJ, Siminoff LA, Kwoh CK. Ethnic differences in the perception of prayer and consideration of joint arthroplasty. Med Care. 2002;40:471–6. doi: 10.1097/00005650-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, Fine MJ. Advancing health disparities research within the health care system: A conceptual framework. Am J Public Health. 2006;96(12):2113–21. doi: 10.2105/AJPH.2005.077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(Suppl 1):S87–94. doi: 10.5435/00124635-200700001-00019. [DOI] [PubMed] [Google Scholar]
- 41.Hausmann LRM, Hanusa BH, Kresevic DM, Zickmund S, Ling BS, Gordon HS, et al. Orthopedic communication about osteoarthritis treatment: does patient race matter? Arthritis Care Res. 2011;63(5):635–42. doi: 10.1002/acr.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen KD, Helmick CG, Schwartz TA, DeVellis RF, Renner JB, Jordan JM. Racial differences in self-reported pain and function among individuals with radiographic hip and knee osteoarthritis: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2009;17(9):1132–6. doi: 10.1016/j.joca.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ang DC, Tahir N, Hanif H, Tong Y, Ibrahim SA. African Americans and Whites are equally appropriate to be considered for total joint arthroplasty. J Rheumatol. 2009;36(9):1971–6. doi: 10.3899/jrheum.081214. [DOI] [PubMed] [Google Scholar]
- 44.Ang DC, Monahan PO, Cronan TA. Understanding ethnic disparities in the use of total joint arthroplasty: application of the health belief model. Arthritis Rheum. 2008;59(1):102–8. doi: 10.1002/art.23243. [DOI] [PubMed] [Google Scholar]
- 45.Ibrahim SA, Hanusa BH, Hannon MJ, Kresevic D, Long J, Kent Kwoh C. Willingness and access to joint replacement among African American patients with knee osteoarthritis: a randomized, controlled intervention. Arthritis Rheum. 2013;65(5):1253–61. doi: 10.1002/art.37899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McIlvane JM, Baker TA, Mingo CA, Haley WE. Are behavioral interventions for arthritis effective with minorities? Addressing racial and ethnic diversity in disability and rehabilitation. Arthritis Rheum. 2008;59(10):1512–8. doi: 10.1002/art.24117. [DOI] [PubMed] [Google Scholar]
- 47.Weng HH, Kaplan RM, Boscardin WJ, Maclean CH, Lee IY, Chen W, et al. Development of a decision aid to address racial disparities in utilization of knee replacement surgery. Arthritis Rheum. 2007;57(4):568–75. doi: 10.1002/art.22670. [DOI] [PubMed] [Google Scholar]
- 48.Borkhoff CM, Wieland ML, Myasoedova E, Ahmad Z, Welch V, Hawker GA, et al. Reaching those most in need: a scoping review of interventions to improve health care quality for disadvantaged populations with osteoarthritis. Arthritis Care Res (Hoboken) 2011;63(1):39–52. doi: 10.1002/acr.20349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1: International Classification of Diseases, 9th Revision, Clinical Modification (ICD9-CM) codes used to determine mental health diagnoses included as covariates
eTable 2: Hazard ratios of all predictors included in adjusted models for full analytic sample and specialty clinic sub-sample


