Table 1.
|
| ||||
|---|---|---|---|---|
| Entry | Reducing Conditions | Sec Intermediate | Electrophile | Notes |
| 1 | LiBEt3H (ref. 17) |
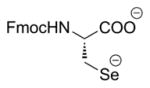
|
|
Trace product formed. Selenol intermediate readily re-formed 1 under the basic reaction conditions. |
| 2 | In(I)I (refs. 21 & 22) |
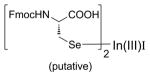
|

|
No product formed. In(III)I diorganyl selenoate unreactive to the tertiary alkyl chloride electrophile. |
| 3a | Zn/HCl (refs. 15 & 16) |
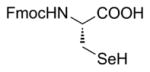
|
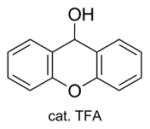
|
84% Fmoc-Sec(Xan)-OH product isolated. |
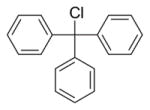
| 3b | Zn/HCl (refs. 15 & 16) |

|

|
87% Fmoc-Sec(Trt)-OH product isolated. |
