Abstract
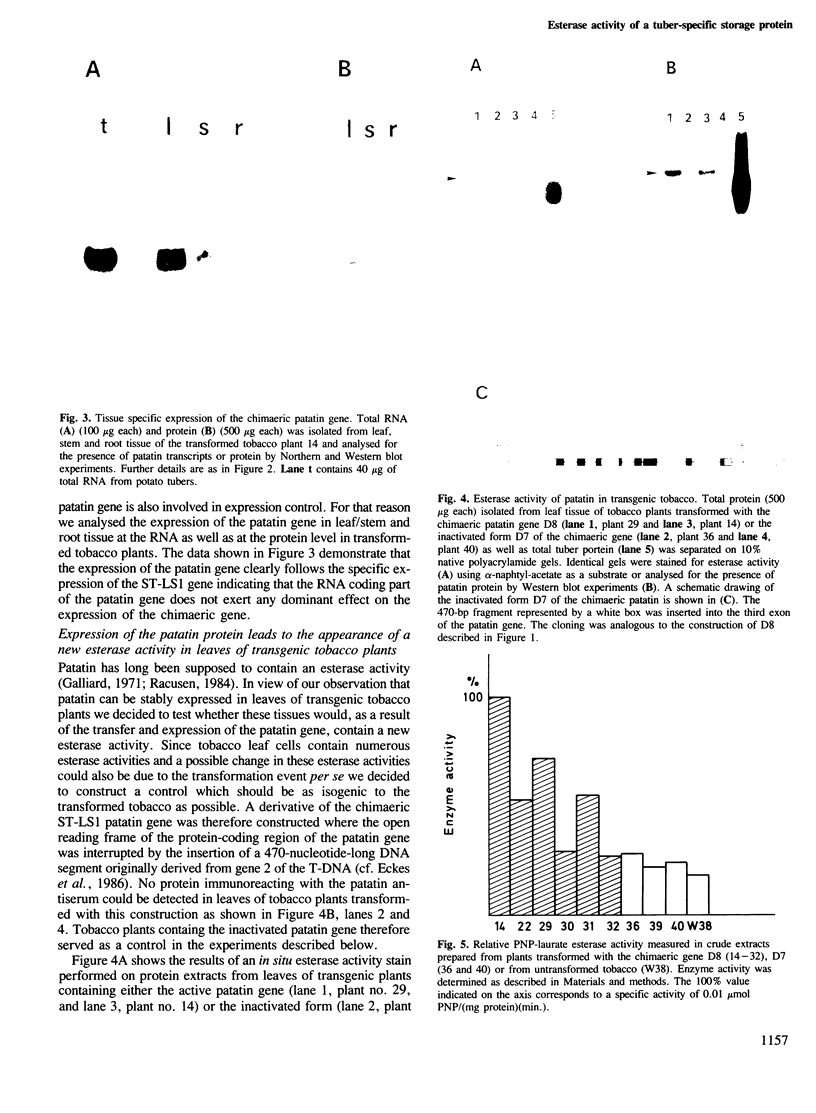
A chimaeric gene composed of the 5' upstream region of STLS1, a leaf/stem specifically expressed gene from Solanum tuberosum, and the RNA-coding as well as the 3' downstream region of patatin, the major storage protein of potato tubers, has been transferred into tobacco plants using the Agrobacterium system. The introduction of this gene led to a leaf/stem specific expression of a 42-kd large protein which immunocrossreacts with patatin antiserum. Only low amounts of immunoreacting protein of smaller size could be detected in transgenic tobacco leaves indicating that the patatin protein is fairly stable in this heterologous environment. The size of the protein as well as the size of the RNA detected in transgenic tobacco leaves using a patatin-specific probe indicates that the patatin RNA was accurately processed in both leaf and stem tissue of tobacco. The expression of the patatin gene led to the appearance of a new esterase activity in the transformed tobacco which co-migrated with a protein immunoreacting with patatin antiserum. These data therefore demonstrate that patatin in addition to serving as a storage protein displays an enzymatic activity.
Keywords: patatin, gene transfer, storage protein, esterase, potato
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Beachy R. N., Chen Z. L., Horsch R. B., Rogers S. G., Hoffmann N. J., Fraley R. T. Accumulation and assembly of soybean beta-conglycinin in seeds of transformed petunia plants. EMBO J. 1985 Dec 1;4(12):3047–3053. doi: 10.1002/j.1460-2075.1985.tb04044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chee P. P., Klassy R. C., Slightom J. L. Expression of a bean storage protein 'phaseolin minigene' in foreign plant tissues. Gene. 1986;41(1):47–57. doi: 10.1016/0378-1119(86)90266-0. [DOI] [PubMed] [Google Scholar]
- Galliard T. The enzymic deacylation of phospholipids and galactolipids in plants. Purification and properties of a lipolytic acyl-hydrolase from potato tubers. Biochem J. 1971 Feb;121(3):379–390. doi: 10.1042/bj1210379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Dunsmuir P., Bedbrook J. High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J. 1985 Oct;4(10):2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matzke M. A., Susani M., Binns A. N., Lewis E. D., Rubenstein I., Matzke A. J. Transcription of a zein gene introduced into sunflower using a Ti plasmid vector. EMBO J. 1984 Jul;3(7):1525–1531. doi: 10.1002/j.1460-2075.1984.tb02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai N., Kemp J. D., Sutton D. W., Murray M. G., Slightom J. L., Merlo D. J., Reichert N. A., Sengupta-Gopalan C., Stock C. A., Barker R. F., Hall T. C. Phaseolin gene from bean is expressed after transfer to sunflower via tumor-inducing plasmid vectors. Science. 1983 Nov 4;222(4623):476–482. doi: 10.1126/science.222.4623.476. [DOI] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F., Morelli G., Fraley R. T., Rogers S. G., Chua N. H. Photoregulated expression of a pea rbcS gene in leaves of transgenic plants. EMBO J. 1985 Dec 1;4(12):3063–3068. doi: 10.1002/j.1460-2075.1985.tb04046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponz F., Paz-Ares J., Hernández-Lucas C., Carbonero P., García-Olmedo F. Synthesis and processing of thionin precursors in developing endosperm from barley (Hordeum vulgare L.). EMBO J. 1983;2(7):1035–1040. doi: 10.1002/j.1460-2075.1983.tb01542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta-Gopalan C., Reichert N. A., Barker R. F., Hall T. C., Kemp J. D. Developmentally regulated expression of the bean beta-phaseolin gene in tobacco seed. Proc Natl Acad Sci U S A. 1985 May;82(10):3320–3324. doi: 10.1073/pnas.82.10.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haute E., Joos H., Maes M., Warren G., Van Montagu M., Schell J. Intergeneric transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of the Ti plasmids of Agrobacterium tumefaciens. EMBO J. 1983;2(3):411–417. doi: 10.1002/j.1460-2075.1983.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]