Figure 5.
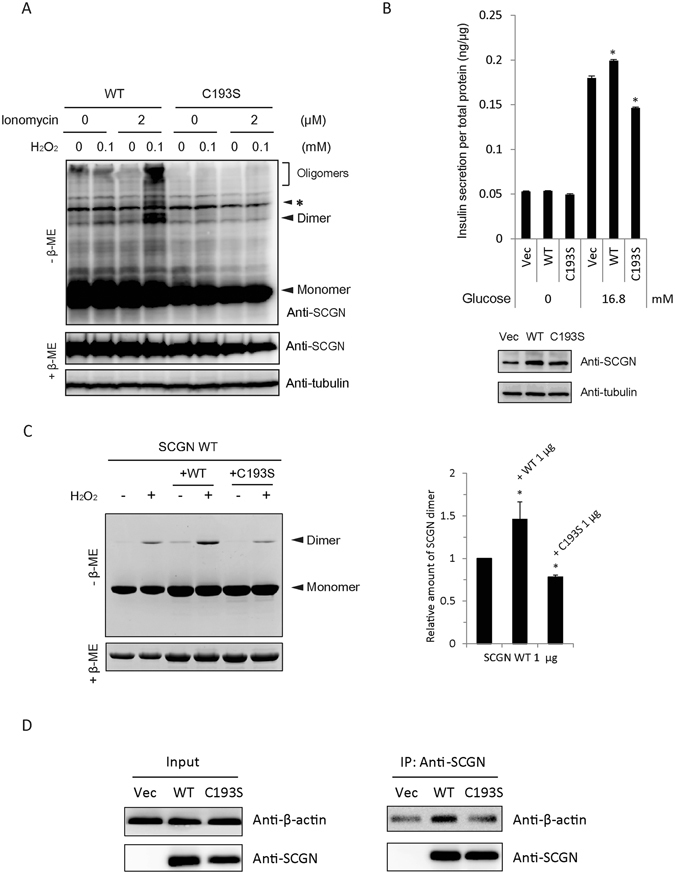
Dimerization of hSCGN is required for its role in insulin secretion. (A) NIT-1 insulinoma cells overexpressing hSCGN WT and C193S mutant were pre-incubated with 0 or 2 μM ionomycin for 5 min followed by incubation with 0 or 100 μM H2O2 for 30 min at 37 °C. Proteins were separated under non-reducing and reducing conditions on SDS PAGE and detected with Western analysis using anti-SCGN antibody. Non-specific bands were indicated as *. (B) NIT-1 cells overexpressing hSCGN WT or C193S mutant were starved with glucose-free HBSS for 2 h followed by stimulation with 16.8 mM glucose for 25 min. The amount of secreted insulin was measured and normalized to total cell protein concentration. Whole cell lysates were subjected to Western analysis with anti-SCGN or anti-α-tubulin antibody. (C) Dimer formations of hSCGN WT, and hSCGN WT mixed with same amount of hSCGN WT or C193S proteins were measured after treating with 2 mM CaCl2 for 15 min at R.T. followed by incubation with 0 or 1 mM H2O2 for 1 h at 37°C. Proteins were detected with coomassie blue-staining (left panel) and were quantified and normalized with reduced form of SCGN (right panel). (D) hSCGN C193S fails to interact with cytoskeletal actin in MDA-MB-231 cells. MDA-MB-231 cells overexpressing SCGN WT and C193S mutant were lysed with a lysis buffer containing protease inhibitors. Cell lysates were immunoprecipitated with anti-SCGN antibody and the immune complexes were separated on SDS-PAGE and detected with western analysis using anti-actin and anti-SCGN antibodies. Data information: In (B,C), data are expressed as the mean ± SD of three experiments (*P < 0.05, Student’s t-test). Full-length gels and blots are presented in Supplementary Fig. S8.
