Abstract
Under steady-state conditions, hematopoietic stem/progenitor cells (HSPCs) egress from bone marrow (BM) and enter peripheral blood (PB) where they circulate at low levels. Their number in PB, however, increases significantly in several stress situations related to infection, organ/tissue damage, or strenuous exercise. Pharmacologically mediated enforced egress of HSPCs from the BM microenvironment into PB is called “mobilization”, and this phenomenon has been exploited in hematological transplantology as a means to obtain HSPCs for hematopoietic reconstitution. In this review we will present the accumulated evidence that innate immunity, including the complement cascade and the granulocyte/monocyte lineage, and the PB plasma level of the bioactive lipid sphingosine-1-phosphate (S1P) together orchestrate this evolutionarily conserved mechanism that directs trafficking of HSPCs.
Keywords: S1P, SDF-1, CXCR4, stem cell mobilization, MAC
Introduction
Hematopoietic stem/progenitor cells (HSPCs) are endowed with enhanced migratory properties. They migrate during organogenesis in the developing mammalian embryo, moving between major anatomical sites where hematopoiesis is initiated and/or is temporarily active (i.e., blood islands in the yolk sac, aortic endothelium, and fetal liver) before they reach their final destination in the developing hematopoietic microenvironment of bone marrow (BM) in the third trimester of gestation [1]. Later in adult life, a small percentage of HSPCs is continuously released from BM niches into the PB, which may be envisioned as a highway by which HSPCs relocate between distant areas of BM in order to keep the total pool of stem cells in remote bone areas in balance [2, 3].
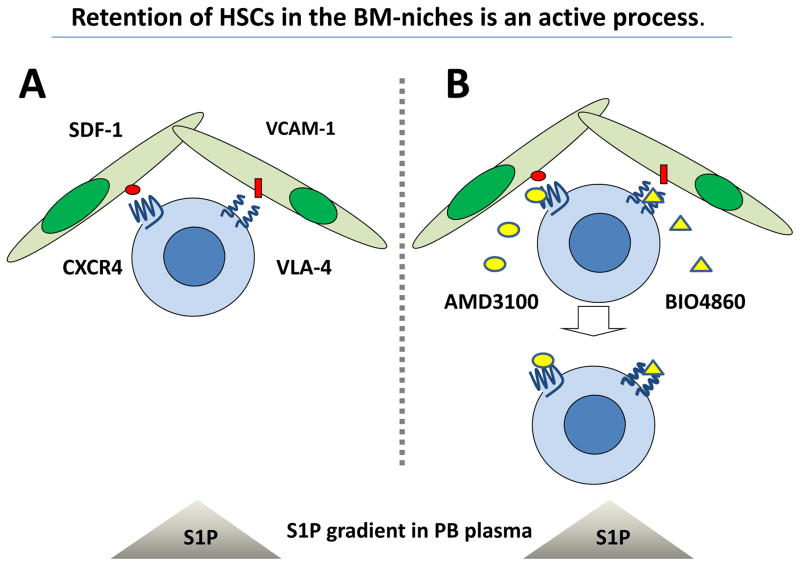
HSPCs reside in BM in hematopoietic stem cell niches, of which the two most important types have been identified [4, 5]. The first type, lining the surface of the bone trabecula, is known as the osteoblastic niche and the second type, located close to endothelial cells in the BM sinusoids, is the so-called endothelial niche. It is postulated that most primitive quiescent stem cells are located in osteoblastic niches and more differentiated cells in endothelial niches. HSPCs residing in BM are retained in these niches by active interaction between ligands expressed in the BM microenvironment and receptors present on the surface of the HSPCs [4, 5]. The most important ligands involved in this process are α-chemokine stromal derived factor-1 (SDF-1) and Vascular Adhesion Molecule-1 (VCAM-1/CD106), which are expressed in hematopoietic niches and interact with their corresponding receptors expressed on HSPCs: the G-protein coupled seven-transmembrane-spanning receptor CXCR4 and the integrin receptor Very Late Antigen-4 (VLA-4/α1β4 integrin), respectively (Figure 1 panel A).
Figure 1. HSPCs are actively retained in BM niches against the S1P chemotactic gradient between PB and BM.
Panel A - HSPCs residing in BM are retained in niches due to active interaction between ligands expressed in the BM microenvironment (SDF-1 and VCMA-1) and their corresponding receptors (CXCR4 and VLA-4) present on the surface of HSPCs. Panel B – Blockage of these retention axes by small-molecule inhibitors (AMD3100 and BIO4860, respectively) exposes HSPCs to the S1P chemotactic gradient between PB and BM and leads to their egress from BM.
As a migratory population of stem cells, HSPCs can easily leave their BM niches and egress into PB where they circulate at low levels, even under steady-state conditions. The number of HSPCs circulating in PB also follows the physiological circadian rhythm of circulation, with a peak in the morning hours and a nadir at night [6–8]. Their number significantly increases in several stress situations as part of danger-sensing mechanisms. Therefore, an increase in HSPCs circulating in PB is observed during inflammation, tissue organ injury (e.g., heart infarct or stroke), hemolytic syndromes (e.g,, hemolytic crisis in sickle cell anemia [SSA] or paroxysmal nocturnal hemoglobinuria [PNH]), and even as a response to strenuous exercise [2, 3, 9–11]. Pharmacologicaly enforced egress of HSPCs from BM into PB is called “mobilization”, where the number of HSPCs circulating in PB increases up to 100 fold. Mobilized HSPCs isolated from PB are currently a preferred source of stem cells for transplantation, because they can be easily harvested from PB by leucopheresis and, what is important from a clinical point of view, they also engraft faster after transplantation than HSPCs harvested from the BM under steady-state conditions [12–14].
Many pharmacological agents that effectively induce mobilization of HSPCs are known. The cytokine granulocyte colony-stimulating factor (G-CSF) is currently the most frequently employed clinical drug, which after a few consecutive daily injections, efficiently mobilizes HSPCs [12]. Mobilization can also be induced in experimental animals within hours after administration of certain chemokines (e.g., interleukin-8 [IL-8], growth-related oncogene protein-beta [Gro-β], or macrophage inhibitory protein-1alpha [MIP-1α]) [2, 15, 16]. Several mobilizing drugs have also been developed to interfere with mechanisms that promote retention of HSPCs in BM niches, including small-molecule antagonists of the chemokine CXCR4 receptor (e.g., AMD3100 or T139) or a small-molecule antagonist of integrin receptor VLA-4 (BIO4860). These small-molecule inhibitors specifically target the SDF-1–CXCR4 and VCAM-1–VLA-4 retention axes, respectively (Figure 1 panel B), and are already successfully employed as mobilizing drugs in the clinic [13, 17, 18].
To obtain more efficient and faster mobilization, some of these compounds can be administered together (e.g., G-CSF with AMD3100 or Gro-β with AMD3100) [19]. Mobilization can also be achieved by administration of some types of polysaccharides (e.g., zymosan or fucoidans), which in animal models have been demonstrated to efficiently mobilize HSPCs within one hour after a single injection [2].
HSPCs are actively retained in BM niches
In addition to the α-chemokine SDF-1–CXCR4 and VCAM-1–VLA-4 (α4β1) axes for retention of HSPCs in BM, mentioned above, there are several other elements that regulate their egress into PB. The most important regulators of HSPC trafficking are proteolytic enzymes, such as matrix metalloproteinases [MMPs], elastase, catepsin-G [12, 20, 21], inhibitors of proteolytic enzymes, tissue inhibitors of metalloproteinases [TIMPs], serpins [3, 22], neural mediators (dopamine and β2-adrenergic receptor agonists) [6–8, 17], elements of the coagulation cascade (e.g., uPAR, thrombin) [17], and, what has been intensively investigated by our team, several cleavage products of the complement cascade (CC) [3, 23, 24].
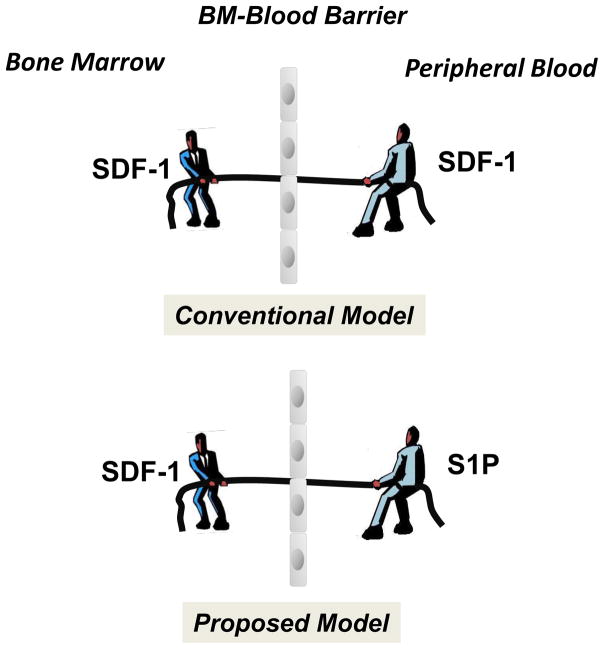
From an historical point of view, SDF-1 was the first potent chemoattractant identified for HSPCs [25, 26], and for many years there was a prevailing concept of a “tug of war” by the SDF-1 gradient between BM and PB that determines whether cells will be released/mobilized from BM into PB or home back from PB to the BM microenvironment (Figure 2). However, changes in the SDF-1 gradient between BM and PB do not always support its having a crucial role in directing egress/mobilization of HSPCs. Specifically, the plasma SDF-1 level does not always correlate with mobilization of HSPCs [27–29]. For example, when employing sensitive ELISA measurement we observed neither significant increases in SDF-1 plasma level during mobilization nor a correlation of its plasma level with the mobilization status of the patients (poor versus good mobilizers) [30]. We are aware, however, that it is impossible at this point to directly measure the SDF-1 level in BM sinusoids, but only indirectly in PB aspirated from major veins.
Figure 2. The concept of a chemotactic tug-of-war gradient between BM and PB explains mobilization and homing of HSPCs.
It has been postulated that an SDF-1 gradient between BM and PB regulates trafficking of HSPCs (homing vs. mobilization). Under steady-state conditions, this gradient across endothelial barrier in BM sinusoids should be in balance. New evidence indicates that, rather than propsoed in conventional model changes in the SDF-1 gradient across the BM–PB barrier (e.g., by an increase of the SDF-1 level in PB), high S1P concentrations in PB plasma provide an opposing chemotactic gradient for HSPCs. However, since HSPCs are retained in BM in hematopoietic niches, the first step in mobilization is their release from these niches and permeabilization of endothelial barrier.
Based on these observations, we became interested in other potential factors that, in addition to SDF-1, could chemoattract HSPCs and thus play a role in their egress from BM. Interestingly while we observed that HSPCs respond robustly by chemotaxis in Transwell chambers to PB plasma, the PB chemotactic activity against HSPCs turned out to be i) insensitive to CXCR4 blockage by AMD3100, ii) unaffected by heat inactivation of plasma that destroys peptides (e.g., SDF-1), but in contrast, iii) highly susceptible to charcoal extraction [30]. This suggested the involvement of lipid components of plasma, and we became interested in the possible involvement of sphingosine-1-phosphate (S1P).
S1P is a bioactive sphingolipid and has been described as a strong chemoattractant for HSPCs [30–36]. It is widely accepted that S1P concentration in PB is much higher than in the BM microenvironment, thus creating a steep chemotactic gradient across the BM–blood barrier (Figure 2) [32, 35]. In addition to free, unbound S1P in plasma, S1P is highly concentrated in red blood cells, which are important transporters for this bioactive lipid, and is associated with PB albumins and high-density lipoproteins (HDL) as well as platelets stored in blood [32, 34, 35].
As we have shown, the high concentration of S1P in PB plasma creates a strong chemotactic gradient for BM-residing HSPCs, even under steady-state conditions [30]. This explains why, in response to small-molecule inhibitors of CXCR4 and VLA-4 (AMD3100 and BIO4860, respectively), HSPCs are relatively easily mobilized into PB (Figure 1B). In further support of this notion, we have shown that AMD3100 mobilization of mouse HSPCs into PB is significantly impaired after the S1P level in the BM microenvironment is upregulated by exposing mice to deoxypriridine (DOP), an antagonist of vitamin B6 that inhibits the S1P-degrading enzyme S1P lyase in tissues [30]. Finally, a role for S1P in mobilization was confirmed in animals that lack one of the key enzymes involved in S1P synthesis. These mice, with low circulating plasma levels of S1P, turned out to be poor mobilizers [36].
Therefore, as shown in Figure 2, S1P creates an active chemotactic gradient between PB plasma and the BM niches in which HSPCs are retained by the active interaction that involves the SDF–CXCR4 and VCAM-1–VLA-4 retention axes. If these retention signals are blocked by, for example, small-molecule inhibitors of CXCR4 or VLA-4, HSPCs egress from BM by following a steep S1P gradient that is continuously present between BM and PB (Figure 1B). As discussed in a later part of this review, this chemotactic S1P gradient may further increase after S1P is released from erythrocytes due to activation of the complement cascade (CC), as observed in G-CSF-induced mobilization or in patients suffering from hemolytic syndromes (e.g., hemolytic crisis in SSA or in PNH patients) [37, 38].
Innate immunity and mobilization of HSPCs
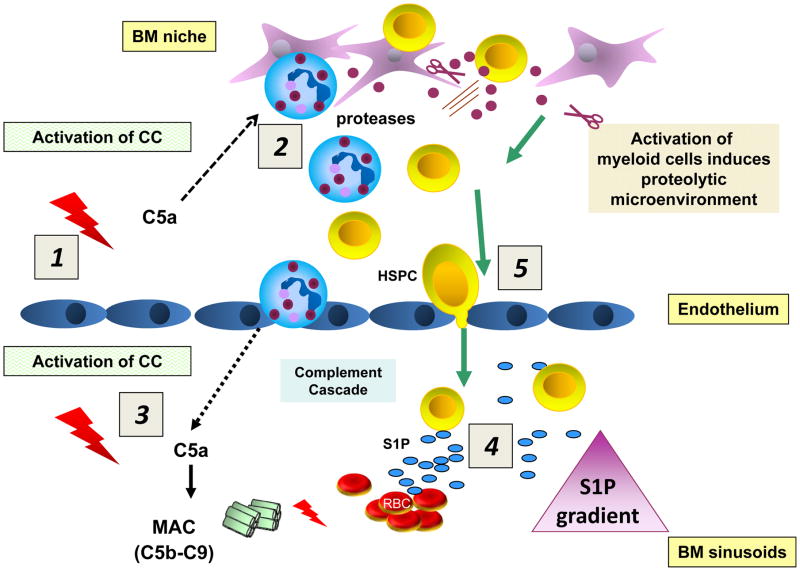
Since mobilization of HSPCs from BM into PB is part of a systemic response to stress (e.g., infections or organ tissue injury) it is not surprising that several elements of innate immunity play an important role in self-defense mechanisms that trigger this phenomenon [3]. For example, we have demonstrated that mobilization of HSPCs is triggered by activation of the CC, which leads to a sequence of events that releases cells from the BM that involves i) generation of CC cleavage fragments (e.g., potent anaphylatoxins, such as C3a, C5a, and C5b-C9, the terminal product of CC activation known as membrane attack complex [MAC]) and ii) activation of granulocytes and monocytes (Figure 3). The roles of these elements of innate immunity and their link to upregulation of the plasma S1P level in the mobilization process will be discussed in more detail below.
Figure 3. Activation of the CC directs mobilization of HSPCs.
HSPCs are actively retained in BM and retention signals in the BM niches counteract an S1P-mediated chemotactic plasma gradient. Activation of the CC in the BM microenvironment (1) leads to generation of C5a, which strongly activates granulocytes and monocytes to release proteolytic enzymes that perturb retention signals for HSPCs in their niches (2). Activation of the CC in BM sinusoids also leads to release of C5a, which chemoattracts granulocytes and monocytes into PB (3). These cells are highly enriched in proteolytic enzymes and are the first to leave the BM and thus “pave the way” for HSPCs that follow in their “footsteps”. At the same time, activation of the CC in the BM sinusoids leads to generation of sublytic and lytic C5b-C9 (MAC), which promotes additional release of S1P from erythrocytes (4). In the final step, HSPCs egress from BM following the higher plasma S1P level in the BM sinusoids (5).
Activation of complement cascade (CC)
As mentioned above, it should not be surprising that mobilization of stem cells is directed by the CC as it is an important and evolutionarily conserved regulatory mechanism for sensing and responding to inflammation and organ injury. Thus, the release of stem cells into the circulation can be envisioned as part of the CC-mediated immune surveillance and response to inflammation and organ/tissue damage. Circulating HSPCs provide cells involved in clearance of pathogens (e.g., granulocytes or macrophages) and cells that regulate the immune response (e.g., dendritic cells) to infected tissues [3, 33]. In addition to HSPCs, other types of BM-residing stem cells can also be mobilized that may play a role in tissue/organ regeneration (e.g., multipotent stromal cells, endothelial progenitors, or very small embryonic-like stem cells) [10, 11, 39].
Work in our laboratory demonstrated that CC activation in BM is triggered by several mobilizing agents, including granulocyte colony stimulating factor (G-CSF), polysaccharides like zymosan, as well as the CXCR4 receptor antagonist AMD3100. As mentioned above, during CC activation, several bioactive cleavage fragments are released (e.g., C3a, desArgC3a, C5a, and desArgC5a anaphylatoxins) and sublytic and lytic C5b-C9 membrane attack complex (MAC) is generated. Activation of the CC during HSPC mobilization was confirmed by i) ELISA detecting C3a and C5a cleavage fragments in plasma, ii) immunofluorescence showing deposition of iC3b on BM stroma and endothelial cells, and iii) histochemical detection of membrane attack complex (MAC) in BM tissue [23, 24, 30].
Interestingly, we observed that CC cleavage fragments affect the retention/mobilization process differently [3]. While C3 cleavage fragments (C3a or desArgC3a) increase retention of HSPCs in BM, C5 fragments (C5a or desArgC5a) enhance their egress into PB. This was evidenced in mobilization studies performed in C3- and C5-deficient animals, which revealed that C3-deficient mice are easy mobilizers [40] and C5-deficient mice are poor mobilizers [41]. These contrasting effects support that retention/mobilization of HSPCs is regulated differently at upstream and downstream levels of CC activation.
The explanation for these opposing effects of C3 and C5 cleavage fragments is that the C3 fragments (C3a and desArgC3a), as we reported, increase/sensitize the responsiveness of HSPCs to SDF-1 homing signals [42], while the C5 fragments (C5a and desArgC5a) play an important role in activation of granulocytes and monocytes in BM to release proteolytic enzymes that attenuate the activity of the SDF-1–CXCR4 and VCAM-1–VLA4 retention axes in BM, while also directly chemoattracting granulocytes and monocytes and thus promoting their egress of from BM into PB (Figure 3) [41]. As will be discussed below in more detail, granulocytes and monocytes play an important role in disintegrating the BM–blood barrier and thus “pave the way” for egress of HSPCs [41].
Finally, activation of the distal part of the CC also leads to generation of lytic or sub-lytic C5b-C9 (MAC), which releases S1P from erythrocytes [34]. Thus, since activation of the CC results in upregulation of red blood cell-derived S1P in plasma, in all these situations in which the CC is activated, the chemotactic attraction of PB plasma for HSPCs increases in parallel.
Involvement of granulocytes/monocytes in mobilization
It is well known that granulocytes and monocytes, which are also important elements of the innate immune system, are required for HSPC mobilization, and the mobilization process is severely impaired in animals that lack these cells [22, 43]. This can be explained by a mechanism in which C5a and desArgC5a released during CC activation stimulate granulocytes to release several proteolytic enzymes into the BM microenvironment that perturb the SDF-1–CXCR4 and VLA-4–VCAM-1 receptor retention signals between HSPCs and their niches (Figure 3). In fact, in a recent interesting report, it has been shown that proteolytic enzymes released from granulocytes directly deplete osteoblasts from osteoblastic niches and thus impair retention of HSPCs [44].
On the other hand, it has been reported that C5a is a strong chemoattractant for granulocytes and monocytes, and these cells are the first to egress from the BM after administration of a mobilizing agent (e.g., G-CSF or AMD3100) [41]. We hypothesize that in doing so granulocytes and monocytes facilitate egress of HSPCs, which follow “in their footsteps” (Figure 3). This proposed mechanism, in which granulocytes permeabilize the sinusoid endothelial barrier, is supported by our recent transmission electron microscopy (TEM) studies [41]. This step is directly regulated by C5a anaphylatoxin, which binds to the G protein-coupled, seven-transmembrane-spanning C5aR present on granulocytes and monocytes. This important role of C5 cleavage fragments in HSPC mobilization explains why C5-deficient mice are poor mobilizers [3, 41].
Activation of the complement cascade and S1P gradient in PB
As mentioned above, we have shown that PB plasma, due to a high level of S1P, exerts a strong chemotactic gradient against BM-residing HSPCs that are actively retained in their niches [4, 5, 45]. In further support of this observation, PB plasma S1P has also been demonstrated to play a pivotal role in egress of lymphoid cells from BM into PB [46–53] and in trafficking of granulocyte-myeloid progenitor cells (CFU-GM) between BM and peripheral tissues [33]. The S1P level, which is already high in PB, may further increase, and there are several potential cellular sources of S1P that may enhance S1P concentration in PB, including, besides erythrocytes, blood platelets and endothelial cells. However, red blood cells seem to be the most abundant source of S1P circulating in plasma [34, 35]. Nevertheless, the S1P plasma level can also be upregulated by release from activated platelets [35]. This explains why the S1P level in PB may increase not only during lysis of erythrocytes but also during activation of platelets as a result of activation of the coagulation cascade.
It is known that both the CC and the coagulation cascade are activated in parallel during infections, tissue injuries, as well as after administration of a mobilizing agent, such as G-CSF [54, 55]. Furthermore, it has been reported that the coagulation cascade may trigger activation of the CC and, vice versa, the CC may activate platelets and coagulation [54]. Thus, both erythrocyte- and platelet-derived S1P may contribute cooperatively to its overall plasma concentration. On the other hand PB plasma level of S1P is also low in mice that have defect in S1P releasing transporter protein in endothelial cells [56] what indicates endothelium as another significant source of S1P circulating in PB..
The release of S1P from erythrocytes exposed to sublytic/lytic MAC and activated platelets contributes to increase of a steep S1P gradient between PB and the BM sinusoids [30]. Therefore, even without changes in the plasma SDF-1 level (the “tug-of-war” mechanism), the S1P gradient is already a crucial executor of HSPC egress from BM into PB (Figure 2). However, it is clear that the S1P gradient alone is not sufficient to allow HSPCs to egress and, in parallel, other granulocyte/monocyte-dependent mechanisms have to be activated that i) release HSPCs from their niches and ii) permeabilize the blood–BM endothelial barrier [9].
The effects of S1P are mediated by five G protein-coupled, seven-transmembrane span receptors (S1P1–S1P5) expressed on HSPCs. While binding of S1P to S1P1 or S1P3 receptors promotes the chemotaxis of HSPCs, activation of the S1P2 receptor has the opposite effect [57]. We observed that S1P strongly chemoattracts HSPCs that reside in BM and lose this activity against HSPCs already mobilized into PB. This could be explained by desensitization of S1P-binding receptors by the high levels of S1P circulating in plasma. A similar S1P receptor downregulation related mechanism has been demonstrated in the case of lymphocytic progenitors released from lymphatic organs and circulating in PB [35, 58–60].
Complement activation, plasma S1P level, and stem cell mobilization in hemolytic syndromes
The mechanisms described above are also highly relevant to mobilization of HSPCs observed in patients suffering from hemolytic syndromes [37, 38]. Based on our data showing a pivotal role of CC activation and S1P plasma level in mobilization of HSPCs, we hypothesized that since erythrocytes release S1P into PB plasma during hemolytic syndromes (acute hemolytic crisis of PNH and SSA), the plasma S1P gradient together with cleavage fragments of the activated CC should promote egress of HSPCs into circulation. In the case of PNH, S1P is released from erythrocytes in a C5b-C9 (MAC)-dependent manner, and, in the case of SSA, from deformed sickle-cell red blood cells. However, since in hemolytic anemias in parallel both complement and coagulation cascades are activated, platelet-derived S1P also contributes to the overall plasma S1P concentration.
The role of S1P-directed hypermobility of HSPCs in Paroxysmal Nocturnal Hemoglobinuria patients
PNH is an uncommon, acquired hemolytic anemia that is often manifested together with hemoglobinuria, abdominal pain, smooth muscle dystonias, fatigue, and thrombosis [61]. PNH results from the expansion of hematopoietic stem cells harboring a mutation in the PIG-A gene, which is required for the biosynthesis of glycosylphosphatidylinositol (GPI-A). This lipid moiety anchors dozens of different proteins to the cell surface, including some involved in resistance to lysis by C5b-C9 (MAC), such as CD55 (Daf1−/−) and CD59. Due to a lack of GPI-A protein, PNH mutation-affected HSPCs also show a defect in cell membrane lipid raft formation and, as a result, show defective adhesion and interaction with BM niches [61–64].
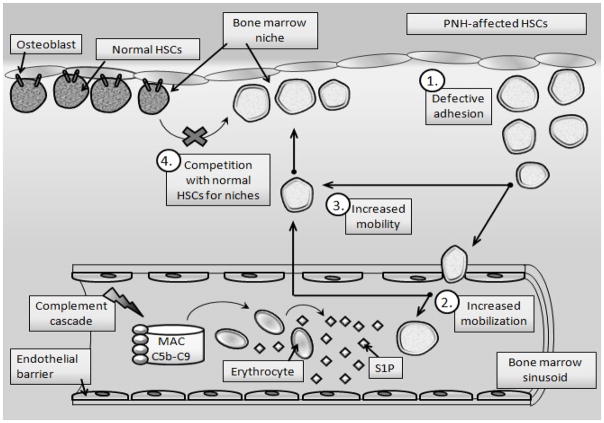
There has been no adequate explanation for why a mutated PNH clone of HSPCs that is highly sensitive to C5b-C9 hemolysis expands over time and outcompetes normal non-mutated HSPCs in BM. However, based on our data connecting activation of the CC, erythrocyte lysis, and release of S1P from red blood cells into PB plasma, we proposed a sequence of events that over time provides an advantage to PNH HSPCs, enabling them to increase at the expense of normal HSPCs [38]. Specifically, based on i) the pivotal role of the complement cascade (CC) in mobilization of HSPCs, ii) the involvement of erythrocyte-derived S1P, which is released from erythrocytes into blood plasma during CC activation, iii) and defective adhesion of PNH affected HSPCs in the BM microenvironment, we hypothesized that PNH is primarily a disorder of defective BM retention and enhanced mobilization of HSPCs into PB. As a result, mutated PNH cells are more motile, accumulate over time in BM, and outcompete and outgrow normal healthy HSPCs, which gradually become displaced by the mutated PNH clone from the BM niches.
Moreover, since the CC is activated during nighttime due to the drop in blood pH [65] as well as during infections, this further explains the exacerbation of PNH symptoms at night (nocturnal hemoglobinuria) [61] in particular in patients that suffer from infection [61]. We therefore propose that PNH in the initial phase of the disorder, is a disease of S1P-mediated increased motility of PNH HSPCs, which gives them a growth advantage over normal HSPCs. With time, this defect in adhesion of PNH HSPCs in the BM microenvironment and their defective interaction with cellular and extracellular elements in the hematopoietic niche may trigger malignant transformation of HSPCs that leads to development of leukemia. Based on the foregoing, Figure 4 illustrates our hypothesis about the involvement of activation of the CC and release of S1P from hemolyzed in C5b-C9-dependent erythrocytes in the pathogenesis of this disorder.
Figure 4. The role of CC activation and S1P-induced hypermobility of PNH-affected HSPCs.
Due to a GPI-A-mediated defect in lipid raft formation, PNH HSPCs have impaired adhesion in BM niches (1), resulting in their enhanced egress (mobilization) from the BM into PB in response to an S1P gradient (2) and enhanced mobility in the BM microenvironment (3). Based on this finding, PNH HSPCs, which show higher motility than their normal healthy counterparts, outcompete and outgrow normal HSPCs in the BM microenvironment over time (4). These cells when complement cascade becomes activated and C5a - that permeabilizes blood-BM barrier is released, are preferentially as we demonstrated mobilized into PB [38]. Eventually, this defect in adhesion of PNH HSPCs in the BM microenvironment and their defective interaction with cellular and extracellular elements in the hematopoietic niche may trigger malignant transformation of HSPCs.
The role of S1P-directed hypermobility of HSPCs in a hemolytic crisis in sickle cell anemia (SSA) patients
It has already been reported that HSPCs are also readily mobilized into PB during hemolytic syndromes in SSA patients [37]. Based on ELISA measurements on PB, it has been proposed that this mobilization is induced by elevated serum levels of stem cell factor (SCF), interleukin-8 (IL-8), granulocyte-colony stimulating factor (G-CSF), and granulocyte-monocyte colony stimulating factor (GM-CSF) [37]. However, none of these factors as it is well known is a chemoattractant for HSPCs.
Therefore, we propose that S1P released from damaged erythrocytes and activated platelets, as in PNH patients, is a major factor involved in egress of HSPCs from BM into PB during hemolytic crisis in SSA patients. Furthermore, since during intravascular hemolysis both the CC and coagulation cascade become activated, this leads to release of S1P from platelets activated by thrombin which contribute to an overall increase of S1P level in PB.
Conclusions
Our recent data provide more evidence that innate immunity and the CC regulate mobilization of HSPCs by modulating the migratory properties of HSPCs, and we propose modulation of the CC as a novel strategy for controlling mobilization of HSPCs. Future studies will address whether the efficiency of CC activation differs between good and poor mobilizers and determine the effect of the CC on cells that are present in the BM microenvironment.
We are aware that in addition to mechanisms described in this review there are other pro-mobilization effects of CC activation involved in egress of HSPCs from BM into PB. Since, functional C3a and C5a receptors have been described on cells lining BM niches, stromal fibroblasts, and endothelial cells in BM sinusoids [66], we envision that CC cleavage fragments (e.g., C3a and C5a) may directly affect permeability of endothelial cells at the BM–blood barrier, modulate the function of BM stromal cells, and directly regulate the function of osteomacs in osteoblastic niches as well as the activity of enzymes involved in synthesis of other bioactive lipids e.g., in eicosanoid metabolism. To support this S1P positively regulates activity of cyclooxygenase-2 that is responsible for synthesis of prostaglandin E2 (PGE2) [30, 67]. PGE2 that is also a bioactive lipid has been demonstrated as potent modulator of HSPC trafficking [68–71]. Thus, we have begun to unravel an important regulatory network involving activation of the CC and bioactive lipids that tightly regulate stem cell trafficking, under both steady-state conditions and stress situations.
Acknowledgments
Supported by NIH grant R01 DK070577 and the Stella and Henry Endowment to MZR.
References
- 1.Mikkola HKA, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–44. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 2.Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood. 2004;103:1580–85. doi: 10.1182/blood-2003-05-1595. [DOI] [PubMed] [Google Scholar]
- 3.Ratajczak MZ, Kim CH, Wojakowski W, Janowska-Wieczorek A, Kucia M, et al. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010;24:1667–75. doi: 10.1038/leu.2010.162. [DOI] [PubMed] [Google Scholar]
- 4.Lévesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–92. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 5.Doan PL, Chute JP. The vascular niche: home for normal and malignant hematopoietic stem cells. Leukemia. 2012;26:54–62. doi: 10.1038/leu.2011.236. [DOI] [PubMed] [Google Scholar]
- 6.Lucas D, Battista M, Shi PA, Isola L, Frenette PS. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell. 2008;4:364–6. doi: 10.1016/j.stem.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simón MF, Andrew C, Miriam M, Paul SF. Circadian rhythms influence hematopoietic stem cells. Current Opinion in Hematology. 2009;16:235–42. doi: 10.1097/MOH.0b013e32832bd0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 9.Ratajczak MZ. Spotlight series on stem cell mobilization: many hands on the ball, but who is the quarterback? Leukemia. 2010;24:1665–6. doi: 10.1038/leu.2010.181. [DOI] [PubMed] [Google Scholar]
- 10.Wojakowski W, Landmesser U, Bachowski R, Jadczyk T, Tendera M. Mobilization of stem and progenitor cells in cardiovascular diseases. Leukemia. 2012;26:23–33. doi: 10.1038/leu.2011.184. [DOI] [PubMed] [Google Scholar]
- 11.Borlongan CV. Bone marrow stem cell mobilization in stroke: a ‘bonehead’ may be good after all! Leukemia. 2011;25:1674–86. doi: 10.1038/leu.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25:211–7. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 13.Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26:34–53. doi: 10.1038/leu.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson D, DeFor T, Burns L, et al. A comparison of related donor peripheral blood and bone marrow transplants: importance of late-onset chronic graft-versus-host disease and infections. Biol Blood Marrow Transplant. 2003;9:52–9. doi: 10.1053/bbmt.2003.50000. [DOI] [PubMed] [Google Scholar]
- 15.Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood. 2004;103:110–19. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- 16.Pelus LM, Fukuda S. Chemokine-mobilized adult stem cells; defining a better hematopoietic graft. Leukemia. 2008;22:466–473. doi: 10.1038/sj.leu.2405021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dar A, Schajnovitz A, Lapid K, et al. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011;25:1286–96. doi: 10.1038/leu.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HM, Wysoczynski M, Liu R, et al. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia. 2010;24:573–82. doi: 10.1038/leu.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King AG, Horowitz D, Dillon SB, et al. Rapid mobilization of murine hematopoietic stem cells with enhanced engraftment properties and evaluation of hematopoietic progenitor cell mobilization in rhesus monkeys by a single injection of SB-251353, a specific truncated form of the human CXC chemokine GRObeta. Blood. 2001;97:1534–42. doi: 10.1182/blood.v97.6.1534. [DOI] [PubMed] [Google Scholar]
- 20.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 21.Levesque JP, Hendy J, Takamatsu Y, et al. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–49. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 22.Pruijt JF, Fibbe WE, Laterveer L, et al. Prevention of interleukin-8-induced mobilization of hematopoietic progenitor cells in rhesus monkeys by inhibitory antibodies against the metalloproteinase gelatinase B (MMP-9) Proc Natl Acad Sci USA. 1999;96:10863–68. doi: 10.1073/pnas.96.19.10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratajczak MZ, Reca R, Wysoczynski M, et al. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18:1482–90. doi: 10.1038/sj.leu.2403446. [DOI] [PubMed] [Google Scholar]
- 24.Reca R, Cramer D, Yan J, et al. A novel role of complement in mobilization: immunodeficient mice are poor granulocyte-colony stimulating factor mobilizers because they lack complement-activating immunoglobulins. Stem Cells. 2007;25:3093–100. doi: 10.1634/stemcells.2007-0525. [DOI] [PubMed] [Google Scholar]
- 25.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–38. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 26.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–94. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 27.Gazitt Y, Liu Q. Plasma levels of SDF-1 and expression of SDF-1 receptor on CD34+ cells in mobilized peripheral blood of non-Hodgkin’s lymphoma patients. Stem Cells. 2001;19:37–45. doi: 10.1634/stemcells.19-1-37. [DOI] [PubMed] [Google Scholar]
- 28.Kozuka T, Ishimaru F, Fujii K, et al. Plasma stromal cell-derived factor-1 during granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. Bone Marrow Transplant. 2003;31:651–54. doi: 10.1038/sj.bmt.1703901. [DOI] [PubMed] [Google Scholar]
- 29.Cecyn KZ, Schimieguel DM, Kimura EY, Yamamoto M, Oliveira JS. Plasma levels of FL and SDF-1 and expression of FLT-3 and CXCR4 on CD34+ cells assessed pre and post hematopoietic stem cell mobilization in patients with hematologic malignancies and in healthy donors. Transfus Apher Sci. 2009;40:159–67. doi: 10.1016/j.transci.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Ratajczak MZ, Lee H, Wysoczynski M, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–85. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura T, Boehmler AM, Seitz G, et al. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004;103:4478–86. doi: 10.1182/blood-2003-03-0875. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Hsu A, Lee JF, et al. To stay or to leave: Stem cells and progenitor cells navigating the S1P gradient. World J Biol Chem. 2011;2:1–13. doi: 10.4331/wjbc.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanel P, Andréani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 35.Obinata H, Hla T. Sphingosine 1-phosphate in coagulation and inflammation. Semin Immunopathol. 2012;34:73–91. doi: 10.1007/s00281-011-0287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golan K, Vagima Y, Ludin A, et al. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood. 2012;119:2478–88. doi: 10.1182/blood-2011-06-358614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamming CE, Augustin L, Blackstad M, et al. Spontaneous circulation of myeloid-lymphoid-initiating cells and SCID-repopulating cells in sickle cell crisis. J Clin Invest. 2003;111:811–9. doi: 10.1172/JCI15956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratajczak J, Kucia M, Mierzejewska K, et al. A novel view of paroxysmal nocturnal hemoglobinuria pathogenesis: more motile PNH hematopoietic stem/progenitor cells displace normal HSPCs from their niches in bone marrow due to defective adhesion, enhanced migration and mobilization in response to erythrocyte-released sphingosine-1 phosphate gradient. Leukemia. 2012 doi: 10.1038/leu.2012.46. (in press) [DOI] [PubMed] [Google Scholar]
- 39.Kucia M, Ratajczak J, Ratajczak MZ. Are bone marrow stem cells plastic or heterogenous--that is the question. Exp Hematol. 2005;33:613–23. doi: 10.1016/j.exphem.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Ratajczak J, Reca R, Kucia M, et al. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004;103:2071–8. doi: 10.1182/blood-2003-06-2099. [DOI] [PubMed] [Google Scholar]
- 41.Lee HM, Wu W, Wysoczynski M, et al. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–62. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reca R, Mastellos D, Majka M, et al. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101:3784–93. doi: 10.1182/blood-2002-10-3233. [DOI] [PubMed] [Google Scholar]
- 43.Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208:251–60. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh P, Hu P, Hoggatt J, Moh A, Pelus LM. Expansion of bone marrow neutrophils following G-CSF administration in mice results in osteolineage cell apoptosis and mobilization of hematopoietic stem and progenitor cells. Leukemia. doi: 10.1038/leu.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 46.Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–8. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 47.Schwab SR, Pereira JP, Matloubian M, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–9. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 48.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 49.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 50.Pereira JP, Xu Y, Cyster JG. A role for S1P and S1P1 in immature-B cell egress from mouse bone marrow. PLoS One. 2010;5:e9277. doi: 10.1371/journal.pone.0009277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thangada S, Khanna KM, Blaho VA, et al. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207:1475–83. doi: 10.1084/jem.20091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allende ML, Tuymetova G, Lee BG, et al. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J Exp Med. 2010;207:1113–24. doi: 10.1084/jem.20092210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juarez JG, Harun N, Thien M, et al. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood. 2012;119:707–16. doi: 10.1182/blood-2011-04-348904. [DOI] [PubMed] [Google Scholar]
- 54.Amara U, Flierl MA, Rittirsch D, et al. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185:5628–36. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spiel AO, Siller-Matula J, Firbas C, et al. Single dose granulocyte colony-stimulating factor markedly enhances shear-dependent platelet function in humans. Platelets. 2010;21:464–9. doi: 10.3109/09537104.2010.485255. [DOI] [PubMed] [Google Scholar]
- 56.Fukuhara S, Simmons S, Kawamura S, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122:1416–26. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cusack KP, Stoffel RH. S1P(1) receptor agonists: Assessment of selectivity and current clinical activity. Curr Opin Drug Discov Devel. 2010;13:481–8. [PubMed] [Google Scholar]
- 58.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 59.Donovan EE, Pelanda R, Torres RM. S1P3 confers differential S1P-induced migration by autoreactive and non-autoreactive immature B cells and is required for normal B-cell development. Eur J Immunol. 2010;40:688–98. doi: 10.1002/eji.200939858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Brodsky RA. How do PIG-A mutant paroxysmal nocturnal hemoglobinuria stem cells achieve clonal dominance? Expert Rev Hematol. 2009;2:353–356. doi: 10.1586/ehm.09.35. [DOI] [PubMed] [Google Scholar]
- 62.Ruiz-Argüelles A, Llorente L. The role of complement regulatory proteins (CD55 and CD59) in the pathogenesis of autoimmune hemocytopenias. Autoimmun Rev. 2007;6:55–61. doi: 10.1016/j.autrev.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 63.Kimberley FC, Sivasankar B, Paul Morgan B. Alternative roles for CD59. Mol Immunol. 2007;44:73–81. doi: 10.1016/j.molimm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 64.Farkas I, Baranyi L, Ishikawa Y, et al. CD59 blocks not only the insertion of C9 into MAC but inhibits ion channel formation by homologous C5b-8 as well as C5b-9. J Physiol. 2002;539(Pt 2):537–45. doi: 10.1113/jphysiol.2001.013381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reis ES, Lange T, Köhl G, et al. Sleep and circadian rhythm regulate circulating complement factors and immunoregulatory properties of C5a. Brain Behav Immun. 2011;25:1416–26. doi: 10.1016/j.bbi.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 66.Ratajczak MZ, Kim CH, Abdel-Latif A, et al. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012;26:63–72. doi: 10.1038/leu.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schraufstatter IU, Discipio RG, Zhao M, Khaldoyanidi SK. C3a and C5a are chemotactic factors for human mesenchymal stem cells, which cause prolonged ERK1/2 phosphorylation. J Immunol. 2009;182:3827–36. doi: 10.4049/jimmunol.0803055. [DOI] [PubMed] [Google Scholar]
- 68.P, Goichberg A, Borodovsky Kalinkovich N, et al. cAMP-induced PKCzeta activation increases functional CXCR4 expression on human CD34+ hematopoietic progenitors. Blood. 2006;107:870–879. doi: 10.1182/blood-2005-03-0941. [DOI] [PubMed] [Google Scholar]
- 69.Hoggatt J, Pelus LM. Eicosanoid regulation of hematopoiesis and hematopoietic stem and progenitor trafficking. Leukemia. 2010;24:1993–2002. doi: 10.1038/leu.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoggatt J, Singh P, Sampath J, et al. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–55. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pelus LM, Hoggatt J. Pleiotropic effects of prostaglandin E2 in hematopoiesis; prostaglandin E2 and other eicosanoids regulate hematopoietic stem and progenitor cell function. Prost & Other Lipid Med. 2011;96:3–9. doi: 10.1016/j.prostaglandins.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]