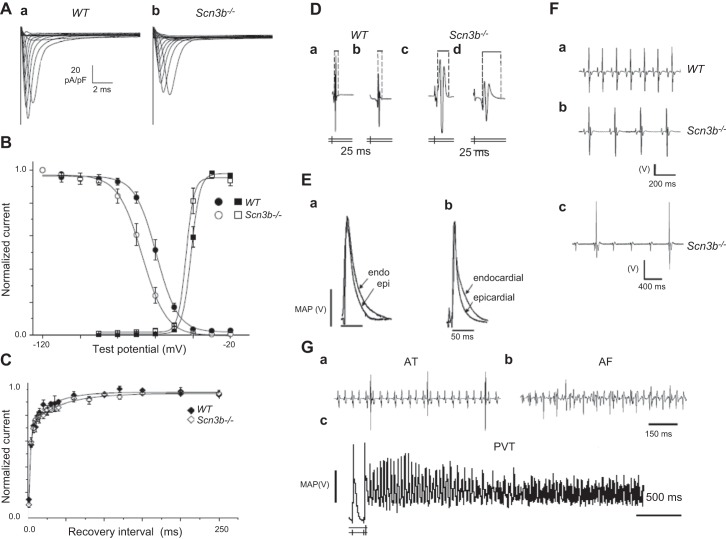
Figure 10.
The Scn3b−/− exemplar for cardiac arrhythmogenesis. A: INa traces from WT (a) and Scn3b−/− myocytes (b) showing peak INa that is significantly smaller in Scn3b−/− myocytes. B: Boltzmann fits to steady-state voltage dependence of activation (squares) and inactivation (circles) in WT (filled symbols) and Scn3b−/− (open symbols). Differing voltage dependence of inactivation in Scn3b−/− compared with WT particularly between holding voltages of −70 and −40 mV. C: recovery from inactivation in WT (filled symbols) and Scn3b−/− (open symbols). D: bipolar electrogram waveforms from programmed electrical stimulation at the longest (a, c) and shortest (b, d) S1–S2 intervals in WT (a, b) and Scn3b−/− hearts (c, d). The latter hearts showed consistently longer waveforms. E: representative monophasic action potential (MAP) recordings from the WT (a) and their shortening in Scn3b−/− hearts (b). F: ECG recordings obtained from WT (a) and Scn3b−/− mice (b). Scn3b−/− mice showed slower heart rates and prolonged PR intervals. c: Some ECGs from Scn3b−/− mice showed ventricular QT complexes occurring independently of regularly occurring atrial P waves, i.e., third degree heart block. G: atrial tachycardia (AT) resulting in regular deflections at a higher frequency (a) and atrial fibrillation (AF) resulting in irregular deflections at a higher frequency following atrial burst pacing (ABP) in Langendorff-perfused Scn3b−/− hearts (b). The ventricular spikes result in the larger, and the atrial spikes in the smaller deflections. c: PES-induced VT beginning as a monomorphic then deteriorating into polymorphic VT in a Scn3b−/− heart preparation. [From Hakim and co-workers (402–404).]