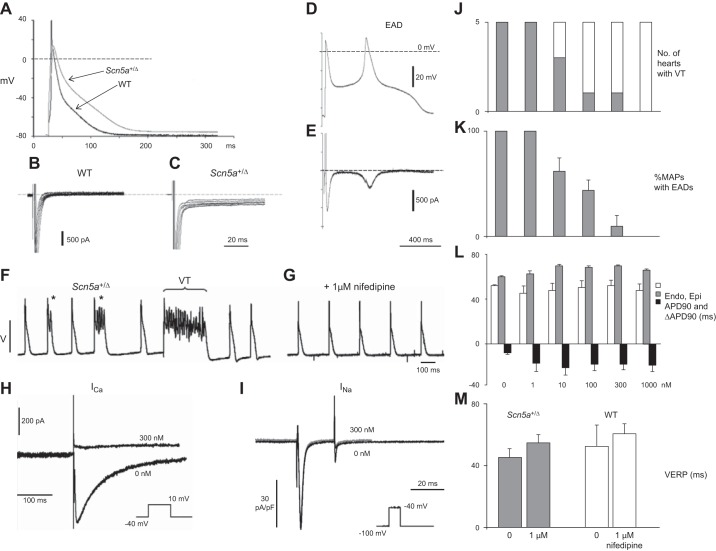
Figure 11.
Separation of contributions to arrhythmogenesis from EADs and transmural APD gradients in Scn5a+/ΔKPQ hearts. A: microelectrode recordings showing action potential prolongation in Scn5a+/ΔKPQ myocytes. B and C: prolonged recovery tail currents after 100 ms test pulses to +40 mV from a −120 mV holding potential in WT (B) and Scn5a+/ΔKPQ myocytes (C). D: action potential (AP) waveforms with early afterdepolarizations (EAD) generating inward late tetrodotoxin (TTX)-sensitive INaL in myocytes in an action potential clamp (E). F and G: epicardial monophasic AP recordings from spontaneously active Scn5a+/ΔKPQ hearts showing multiple EADs and nonsustained VT (F), abolished by 1 μM nifedipine (G). H and I: patch-clamp studies in isolated LV ventricular myocytes from Scn5a+/ΔKPQ hearts demonstrating that nifedipine (300 nM) completely suppressed the inward Ca2+ current following depolarizing steps from a holding voltage of −40 mV to 10 mV (H). In contrast, nifedipine had no effect on inward Na+ currents in response to depolarizing steps from −100 mV to −40 mV (I). J–L: number of hearts showing VT arrhythmia (J), percentage of monophasic action potentials showing EADs in Scn5a+/ΔKPQ hearts (K), and mean ± SE endocardial and epicardial APD90 values and ΔAPD90 in Scn5a+/ΔKPQ (L) at different nifedipine concentrations (0 nM, 1 nM, 10 nM, 100 nM, 300 nM, and 1 μM). M: effects of nifedipine (1 μM) on VERPs of Scn5a+/ΔKPQ and WT hearts. [From Head et al. (416) and Thomas et al. (1128).]