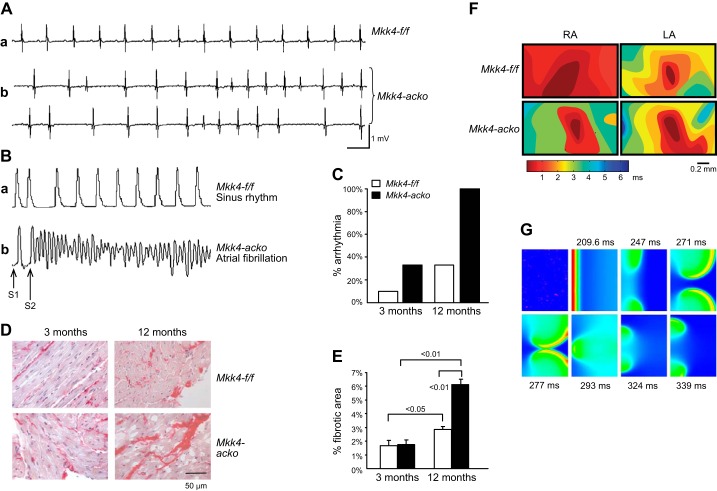
Figure 21.
The arrhythmic mitogen-activated protein kinase kinase 4 knockout heart as an arrhythmic exemplar for fibrotic change. In vivo and ex vivo cardiac electrophysiological characterizations of mice carrying an atrial cardiomyocyte specific mitogen-activated protein kinase kinase 4 knockout, Mkk4-acko, compared with Mkk4-flox/flox, Mkk4-f/f, controls. A: representative in vivo ECG recordings showing (a) normal rhythm in Mkk4-f/f (a) in contrast to polymorphic atrial ectopic beats and spontaneous atrial tachycardic episodes in Mkk4-acko mice (b). B: ex vivo atrial epicardial monophasic action potential (AP) recordings in Langendorff-perfused hearts during programmed electrical stimulation interposing extrasystolic S2 stimuli following trains of pacing S1 stimuli. These show contrasting persistent sinus rhythm in Mkk4-f/f (a) with observations of frequent AF in Mkk4-acko (b). C: occurrence of atrial arrhythmic events (AT and AF) in young (3 mo) and old (12 mo), Mkk4-f/f and Mkk4-acko, hearts. D: picrosirius red-stained atrial tissue, fibrotic areas dark red, from 3- and 12-mo-old, Mkk4-f/f and Mkk4-acko, mice. E: percentage fibrotic area in 3- and 12-mo-old Mkk4-f/f (white) and Mkk4-acko atria (black bar). F: epicardial multielectrode array (MEA) activation maps resulting from differing AP conduction velocities following pacing in the center of the array in right (RA) and left atria (LA) of Mkk4-f/f and Mkk4-acko mice (a). G: computer modeling of the effects of fibroblast-cardiomyocyte coupling resulting in reentry following a premature beat. A standard S1S2 stimulation protocol is applied at the left edge of the 2D model containing randomly distributed fibroblast populations in which between one and five fibroblasts are coupled to any given cardiomyocyte. Subsequent snapshots demonstrate a breaking down of the wavefront of atrial excitation wave leading to formation of reentrant excitation waves. [From Davies et al. (245).]