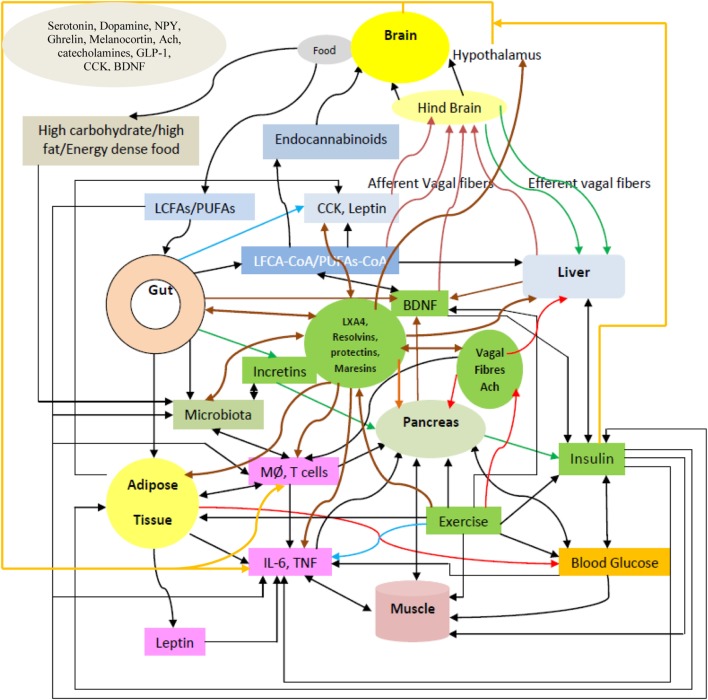
Figure 12.
Scheme showing relationship among diet, gut microbiota, vagus, exercise, polyunsaturated fatty acid (PUFAs), lipoxins (LXs), resolvins, protectins, and maresins, and blood glucose, insulin, and tissues/organs concerned with glucose homeostasis: pancreas, muscle, liver, adipose tissue, and brain. High calorie diet induces low-grade systemic inflammation, obesity, and insulin resistance. PUFAs decrease insulin resistance, suppress secretion of pro-inflammatory cytokines, and lead to the formation of (a) LCFAs-CoA; (b) enhance gut cholecystokinin (CCK) secretion; and (c) augment endocannabinoids formation that act via afferent vagal fibers on hypothalamus to induce satiety and decrease appetite. PUFAs lead to increase in the formation of LXs, resolvins, protectins, and maresins that reduce insulin resistance, protect β cells from toxins, inhibit IL-6 and tumor necrosis factor-α (TNF-α) production, augment brain-derived neurotrophic factor (BDNF) production and action, interact with incretins, CCK, and acetylcholine, and influence gut microbiota, and may act on hypothalamic neurons and modulate insulin response of hypothalamic neurons and enhance response of peripheral tissues to insulin (reduce insulin resistance) and, thus, ameliorate type 2 DM. PUFAs enhance the growth of Bacteroidetes and inhibit Firmicutes and, thus, reduce obesity. PUFAs may augment the production and secretion of gut incretins that, in turn, augment insulin secretion. PUFAs enhance BDNF production, which inhibits appetite and decrease obesity. Liver and pancreas talk with each other through vagal fibers. Exercise reduces insulin resistance and obesity by (i) suppressing production of pro-inflammatory cytokines; (ii) increasing BDNF production in the brain and enhanced levels in the plasma; (iii) enhancing the production of lipoxin A4 (and possibly that of resolvins, protectins, and maresins) from muscle and gut; (iv) upregulating glucose utilization; (v) increasing vagal tone and thus, is (vi) anti-inflammatory in nature. Adipose tissue of obese subjects is infiltrated by macrophages and lymphocytes that secrete high amounts of IL-6 and TNF-α that cause low-grade systemic inflammation resulting in insulin resistance. Leptin has pro-inflammatory actions. Bacteroidetes, the predominant bacteria in the gut of the lean subjects, whereas Firmicutes are dominant in the gut of obese. Firmicutes breakdown polysaccharides and, thus, provide higher amounts of energy source that enhances the probability of development of obesity. Firmicutes stimulate gut associated lymphocytes and macrophages and augment production of pro-inflammatory cytokines. Insulin has anti-inflammatory actions and, hence, is likely that hyperinsulinemia seen in obesity and type 2 DM could be a compensatory phenomenon in order to suppress low-grade systemic inflammation seen in them. Insulin enhances activity of desaturases that leads to increased formation of arachidonic acid, eicosapentaneoic acid and docosahexaenoic acid, the precursors of LXs, resolvins, protectins, and maresins. Though expression and genotype (including single nucleotide polymorphism) of UCPs, FOXC2, adiponectin, FTO, MC4R, and other related genes are closely associated with obesity, their expression and function is modulated by diet, exercise, and other life-style-related factors. Thus, a close interaction(s) exists among genes, gut, diet, microbiota, and exercise that is not only complex but also interesting in the pathogenesis of obesity and type 2 DM. It may be noted here that PUFAs and acetylcholine and exercise can influence production and action of various neurotransmitters, such as serotonin, dopamine, leptin, ghrelin, GABA, and α-MSH and, thus, muscle, gut, food, and brain interact with each other and determine development/amelioration/prevention of obesity and type 2 DM.