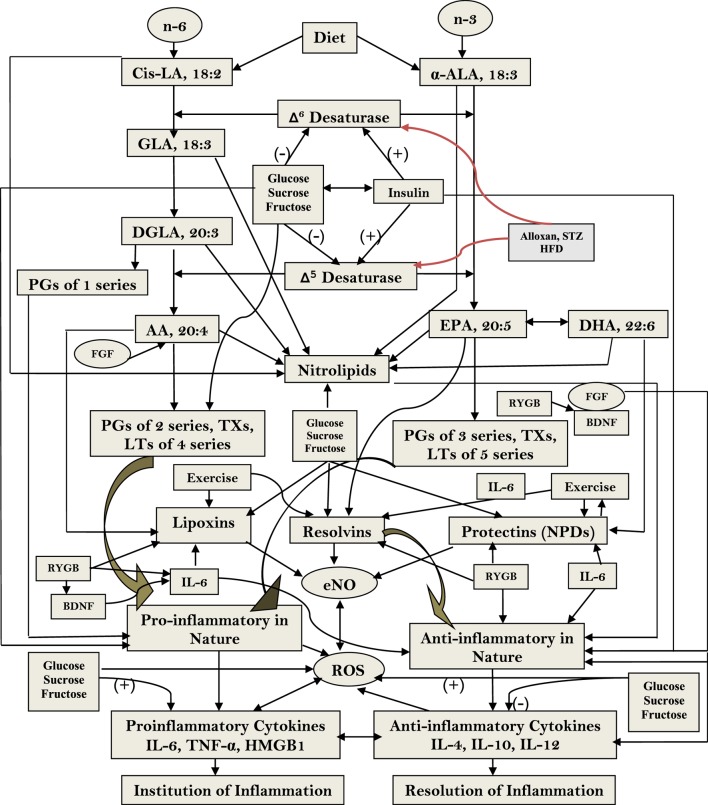
Figure 3.
Scheme showing the metabolism of essential fatty acids, their role in inflammation and the effect of glucose, sucrose, and fructose on the activities of desaturases and formation of various eicosanoids, cytokines, and lipoxins (LXs), resolvins, and protectins. (+) Indicates increase in the activity or enhanced formation. (−) Indicates decrease in the activity or decreased formation. Glucose, sucrose, and fructose decrease activities of Δ6 and Δ5 desaturases and, thus, decrease the formation of arachidonic acid (AA), eicosapentaneoic acid (EPA), and docosahexaenoic acid (DHA) that are precursors of various eicosanoids and LXs, resolvins, and protectins. Glucose, sucrose, and fructose seem to enhance the formation of pro-inflammatory prostaglandins, leukotrienes, and thromboxanes and generation of free radicals and decrease the formation of LXs, resolvins, and protectins that have anti-inflammatory activities and prevent development of type 2 diabetes mellitus and metabolic syndrome and insulin resistance; they may also enhance the formation of pro-inflammatory cytokines and decrease those of anti-inflammatory cytokines. The pro-inflammatory activities of glucose, fructose, and sucrose may be in the order of fructose > sucrose ≥ glucose. Nitrolipids are formed due to interaction between polyunsaturated fatty acids and nitric oxide and these compounds have anti-inflammatory activity. Fibroblast growth factor 1 (FGF1) is a critical transducer of remodeling of adipose tissue in response to fluctuations in nutrient availability that is essential for maintaining metabolic homeostasis and is regulated by the nuclear receptor peroxisome proliferator-activated receptor-γ (PPAR-γ). PPAR-γ is an adipocyte master regulator and the target of the thiazolidinedione class of insulin-sensitizing drugs. FGF1 is the prototype of the 22-member FGF family of proteins and is involved in a range of physiological processes, including development, wound healing, and cardiovascular changes. FGF1 is highly induced in adipose tissue in response to a high-fat diet and that mice lacking FGF1 develop an aggressive diabetic phenotype coupled to aberrant adipose expansion when challenged with a high-fat diet. FGF1-deficient mice have abnormalities in the vasculature network, an accentuated inflammatory response, aberrant adipocyte size distribution, and ectopic expression of pancreatic lipases. It is interesting that withdrawal of the high-fat diet, inflamed adipose tissue fails to properly resolve, resulting in extensive fat necrosis that could be attributed to decreased production of LXs, resolvins, protectins, and maresins. Adipose induction of FGF1 in the fed state is regulated by PPAR-γ acting through a conserved promoter proximal PPAR response element within the FGF1 gene. These results suggest that the PPAR-γ–FGF1 axis is critical for maintaining metabolic homeostasis and insulin sensitization (48). In this context, FGF-19 has been shown to have hypoglycemic actions. Central nervous system responds to FGF-19 administered in the periphery. In mouse models of insulin resistance, leptin-deficiency and high-fat diet feeding and intracerebroventricular infusions of FGF-19 improved glycemic status, reduced insulin resistance and potentiated insulin signaling in the periphery. In addition, central action of FGF-19 included suppression of AGRP/neuropeptide Y neuronal activity (49). Furthermore, high-fat diet (HFD)-fed mice lacking lysosome-associated membrane protein-2 (lamp-2), which is essential for the fusion with lysosome and subsequent degradation of autophagosomes, showed a resistance against HFD-induced obesity, hyperinsulinemic hyperglycemia, and tissue lipid accumulation, accompanied with higher energy expenditure due to high expression levels of thermogenic genes in brown adipose tissue in HFD-fed lamp-2-deficient mice. Serum level of FGF-21 and its mRNA expression level in the liver were significantly higher in HFD-fed lamp-2-deficient mice in an ER stress-, but not PPAR-α-, dependent manner. These results suggest that a lamp-2-dependent fusion and degradation process of autophagosomes, and FGF-21 are involved in the pathogenesis of diabetes implicating a role for autophagy in this process (50). FGF activates phospholipases (51–53) that leads to the release of polyunsaturated fatty acid (PUFAs) that, in turn, can be utilized for the formation of various eicosanoids, LXs, resolvins, protectins, and maresins. Thus, PUFAs and LXs resolvins, protectins, and maresins could mediate anti-obesity and antidiabetic actions of FGFs. Alloxan, streptozotocin, and HFD block the activity of Δ6 and Δ5 desaturases and, thus, lead to a decrease in the synthesis and plasma and tissue levels of GLA, DGLA, AA, EPA, and DHA and decreased formation of LXs, resolvins, protectins, and maresins (from AA, EPA, and DHA) that could lead to increase in inflammation [increase in IL-6 and tumor necrosis factor-α (TNF-α)] and failure of resolution of inflammation and tissue repair. This may result in increase in peripheral insulin resistance, inflammation of mesenteric tissue, gut, adipose tissue, and liver (including NAFLD = non-alcoholic fatty liver disease). It may also lead to inflammation of hypothalamic neurons.