Table 1.
Halide Inhibition in Conjunctive Coupling.a
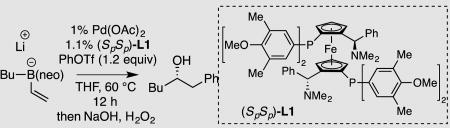
| |||
|---|---|---|---|
|
| |||
| entry | alteration | yieldb | erc |
| 1 | none | 77 | 98:2 |
| 2 | 1% LiI | 13 | 98:2 |
| 3 | 1% LiBr | 41 | 98:2 |
| 4 | 1% LiCl | 40 | 98:2 |
| 5 | 1% (n-Bu)4NCl | 31 | 98:2 |
| 6 | 1% LiI, 5% catalyst | 69 | 98:2 |
| 7 | PhCl instead of PhOTf | <5 | nd |
| 8 | PhBr instead of PhOTf | 9 | 96:4 |
| 9 | PhI instead of PhOTf | 9 | 96:4 |
| 10 | 100% LiBr | 23 | 22:78 |
| 11 | 100% n-Bu4NBr | 19 | 92:8 |
The "ate" complex was prepared by addition of n-butyllithium to vinylB(neo) and the conjunctive coupling was conducted at 0.17 M.
Yield represents isolated yield of purified material.
Enantiomer ratio (er) determined by chiral SFC analysis.
