Table 3.
Effect of solvent on catalytic conjunctive coupling reactions.a
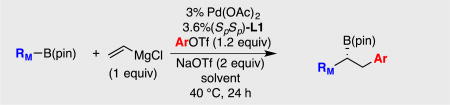
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | RM | Ar | solvent | yieldb | erc |
| 1 | 3-butenyl | Ph | THF | <5% | n/a |
| 2 | 3-butenyl | Ph | THF/DMSO | 73 | 91:9 |
| 3 | Ph | Ph | THF | 72 | 94:6 |
| 4 | Ph | Ph | THF/DMSO | 81 | 96:4 |
| 5 | Ph | p-MeO-Ph | THF | 78 | 92:8 |
| 6 | Ph | p-MeO-Ph | THF/DMSO | 89 | 98:2 |
| 7 | Ph | p-CF3-Ph | THF | 67 | 75:25 |
| 8 | Ph | p-CF3-Ph | THF/DMSO | 85 | 95:5 |
Reactions conducted as described in the text (see SI for additional details) and the conjunctive coupling was conducted at 0.17 M.
Yield represents isolated yield of purified material.
Enantiomer ratio (er) determined by chiral SFC analysis of boronic ester.
