Figure 1. An inducible CENP-A replacement strategy enables functional and selective inactivation of the Y chromosome centromere in human cells.
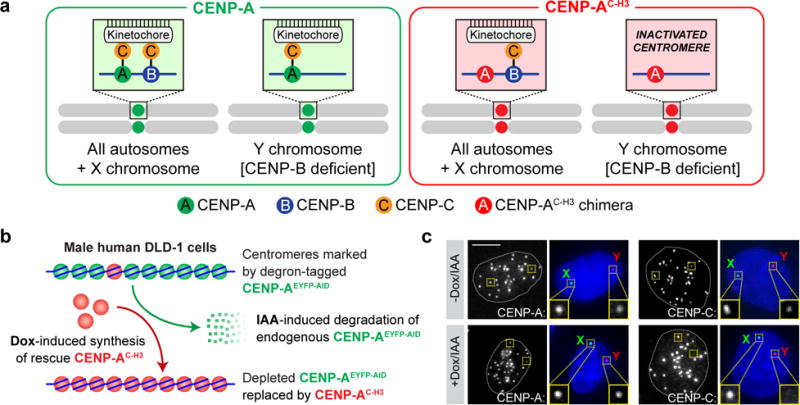
(a) Schematic depicting the strategy used to functionally inactivate the CENP-B-deficient Y centromere with a CENP-A chimera containing the carboxy-terminal tail of histone H3 (CENP-AC-H3) that cannot directly recruit CENP-C. All other chromosomes assemble kinetochores through the CENP-B-dependent pathway. (b) Schematic depicting the approach for inducible replacement of endogenous CENP-A (green circles) at all centromeres with CENP-AC-H3 (red circles). Doxycycline (dox) induces transcriptional synthesis of the rescue CENP-AC-H3 chimera and auxin (IAA) triggers rapid degradation of the endogenous, auxin-inducible degron (AID)-tagged CENP-A protein. (c) Immuno-FISH images of DLD-1 cells rescued with CENP-AC-H3 following 24h dox/IAA treatment. DNA FISH was used to spatially identify the locations of the X (green) and Y (red) centromeres in DAPI-stained interphase nuclei combined with immunofluorescence for detection of CENP-A or CENP-C. Images are representative of 2 independent experiments. Scale bar, 5 μm.
