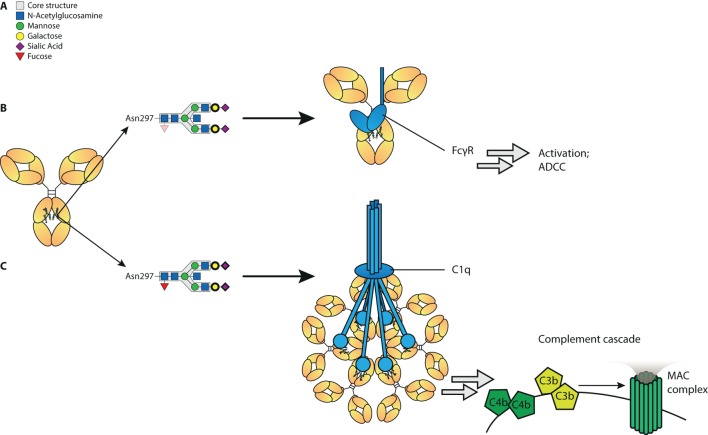
Figure 6.
Proposed model of influence of immunoglobulin G (IgG)-Fc glycan composition on effector functions. (A) Standard composition of bi-antennary Fc-glycan, (B) Afucosylation of IgG-Fc glycan increases binding affinity to FcγRIII and subsequent antibody-mediated functions, such as antibody-dependent cellular cytotoxicity (ADCC). In addition galactosylation further increases affinity to FcγRIII and function of afucosylated IgG. (C) Galactosylation enhances binding of IgG to complement component C1q and activation of the classical complement pathway, which results in cleavage of complements C4, C3, and further initiation of the membrane attack complex (MAC). Sialylation may further increase C1q binding and complement activation. Glycan residues that need to be present to enhance indicated effector function (ADCC/complement-dependent cytotoxicity) are displayed with bolder lines, and for those that need to be absent to enhance indicated effector functions are displayed with faded colors.