Abstract
Neural induction is the process through which pluripotent cells are committed to a neural fate. This first step of Central Nervous System formation is triggered by the "Spemann organizer" in amphibians and by homologous embryonic regions in other vertebrates. Studies in classical vertebrate models have produced contrasting views about the molecular nature of neural inducers and no unifying scheme could be drawn. Moreover, how this process evolved in the chordate lineage remains an unresolved issue. In this work, by using graft and micromanipulation experiments, we definitively establish that the dorsal blastopore lip of the cephalochordate amphioxus is homologous to the vertebrate organizer and is able to trigger the formation of neural tissues in a host embryo. In addition, we demonstrate that Nodal/Activin is the main signal eliciting neural induction in amphioxus, and that it also functions as a bona fide neural inducer in the classical vertebrate model Xenopus. Altogether, our results allow us to propose that Nodal/Activin was a major player of neural induction in the ancestor of chordates. This study further reveals the diversity of neural inducers deployed during chordate evolution and advocates against a universally conserved molecular explanation for this process.
The first developmental step in the formation of the vertebrate Central Nervous System (CNS) is called neural induction. It is the instructive process by which naive ectodermal cells are committed to a neural fate. The concept of neural induction was established by Hilde Mangold and Hans Spemann. They showed that the dorsal blastopore lip of a newt gastrula, when grafted to the ventral side of a host gastrula, was able to induce the formation of a Siamese twin embryo in which the secondary CNS developed from the host and not from the graft 1. This embryonic territory with inductive capacities was called the organizer and many efforts have been dedicated to understand the nature of the neural inductive signals emanating from this structure. In the first molecular model of neural induction, called the "default model", it was proposed that ectodermal cells become epidermal when exposed to Bone Morphogenetic Protein (BMP) signals and neural when deprived of BMP and of any other signals 2. In the early vertebrate embryo, the BMP signalling pathway is active in the ventral region, whereas the dorsal organizer produces BMP antagonists that act as neural inducers 3–5. However, this model has been challenged by various studies suggesting that Fibroblast Growth Factor (FGF) signalling also contributes directly to neural induction in Xenopus and chick 6–11.
If the mechanisms controlling neural induction in vertebrates are subject to debate, their evolutionary origins are even more obscure. In tunicates, the sister group of vertebrates, the embryo lacks an organizer and inhibition of the BMP signal is not required for the formation of the CNS 12, whereas the FGF signal is indispensable 13. Therefore, understanding how the CNS develops in cephalochordates (i.e. amphioxus), the sister group of all remaining chordates, may shed light on the ancestral mechanisms controlling neural induction in this lineage. Graft experiments 14 and gene expression data 15 have suggested that the amphioxus dorsal blastopore lip could be homologous to the vertebrate organizer. Additionally, it has been shown that activation of the BMP signalling pathway ventralizes the amphioxus embryo, leading to loss of the neural plate 15–17. However, experiments undertaken in order to inhibit this signal resulted in modest expansion of axial neural plate gene expression, calling into question the applicability of the default model 18. On the other hand, inhibition of the FGF signalling pathway does not suppress neural induction 19, supporting the idea that FGF is not unconditionally required. Interestingly, activation of the Nodal/Activin pathway in amphioxus leads to complete dorsalization of the embryo in which the whole ectoderm expresses neural genes 16. However, the precise mode of action of Nodal in ectoderm neuralization remains to be elucidated. In vertebrates, Nodal is a key signal produced by the organizer, which acts as a mesendoderm inducer and controls gastrulation movements 20. While these early functions of Nodal signalling were deeply studied, its putative role during neural induction remains to be addressed.
Study of neural induction in amphioxus has been hampered by the lack of appropriate experimental setups. To overcome this fundamental problem, we developed micro-surgery to obtain naive ectodermal explants. Using graft experiments combined to molecular analyses, we show that the dorsal blastopore lip of amphioxus is a functional organizer able to promote the formation of a secondary body axis and the acquisition of neural fate by naive ectodermal cells. We also demonstrate that Nodal/Activin is the main neural induction signal emanating from the organizer in amphioxus. Finally, we show that this pathway also participates to neural induction in Xenopus.
Amphioxus organizer
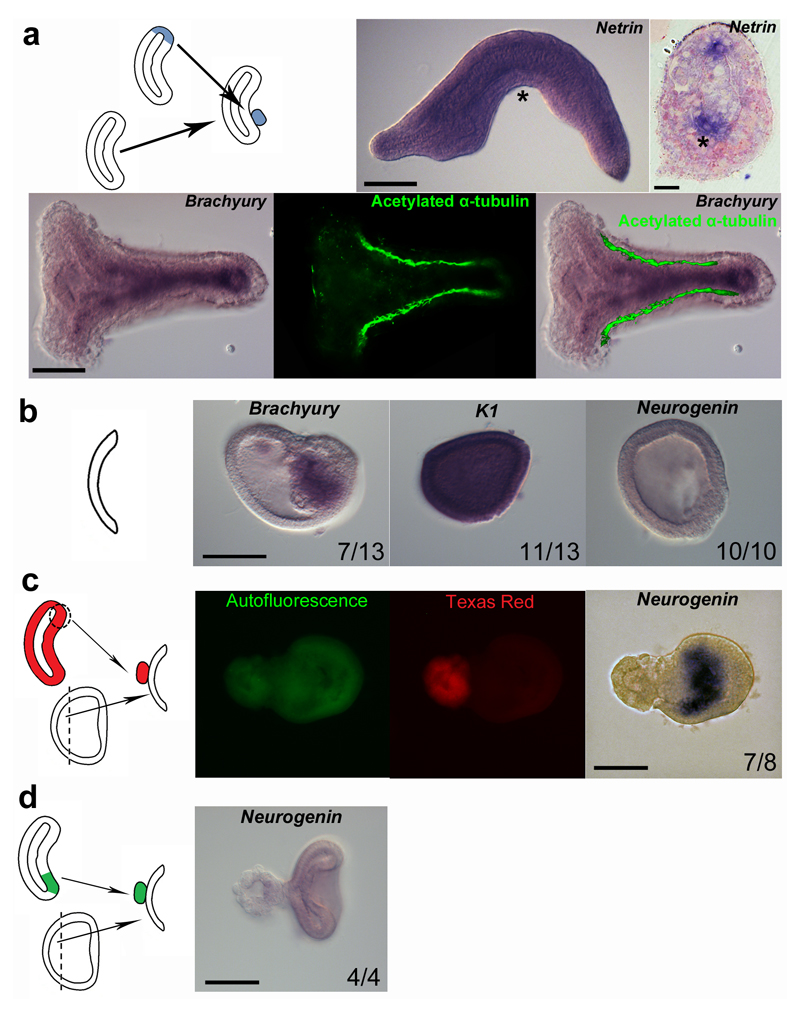
If previous studies 14–15 suggested that the dorsal blastopore lip of amphioxus may be homologous to the vertebrate organizer, there was no direct evidence demonstrating that it is able to trigger neural induction. To address this question, we first reproduced old grafting experiments 14 . We grafted the dorsal lip of the blastopore of early gastrula (G2) stage embryos onto the ventral side of the archenteron of host embryos (Fig 1a). Grafted embryos showed partial secondary axis formation (Fig. 1a), as previously described 14, or complete secondary axis development (Fig. 1a). We then explored the putative neural inductive capacity of the dorsal blastopore lip on naive ectoderm. Ectoderm explanted from G1 gastrulae (Gastrula Explant, GE) developed into blastula shaped hollow balls consisting of a single cell layer, or of an outer single cell layer associated with an inner cell mass, as previously observed 21. The inner cell mass acquired a mesodermal fate as indicated by Brachyury expression (Fig. 1b). The external cell layer developed entirely into epidermis as revealed by the expression of Keratin 1 (K1, Fig. 1b and Supplementary Fig. 1). Moreover, no Neurogenin expression could be detected (Fig. 1b), regardless of the presence or not of Brachyury-positive cells, showing that GE developed by default into epidermis. To ascertain the inductive capacities of the organizer, we grafted the mesendodermal part of the dorsal blastopore lip of fluorescent embryos onto GE (Fig. 1c). In grafted GE, Neurogenin was broadly expressed exclusively in explant cells, indicating that host cells received a neural inducing signal produced by the graft (Fig. 1c). In contrast, we never observed Neurogenin expression when we recombined GE and ventral blastopore lip (Fig. 1d).
Figure 1. The dorsal blastoporal lip of amphioxus is homologous to the vertebrate organizer.
a, Graft experiments were undertaken by dissecting and inserting the blastopore lip of a G2 gastrula stage embryo in the ventral archenteron of a host embryo at the same developmental stage. Some embryos showed partial double axis as indicated using the neural marker Netrin. Other embryos formed a complete double axis as indicated by expression of the notochordal marker Brachyury and by immunostaining of axons for acetylated α-tubulin. The arrowhead indicates the position of the transverse section and the asterisks the ectopic expression of Netrin. b, Ectodermal explants were obtained through micro-dissection of the animal pole of G1 gastrula (gastrula explants, GE). Half of the ectodermal explants showed an inner cell mass expressing the mesoderm marker Brachyury. The external cell layer expressed the epidermal marker K1, whereas no expression of the neural marker Neurogenin was detected. c, Graft on GE of a fluorescently labelled (Texas Red) dorsal blastoporal lip induced the expression of Neurogenin exclusively in host cells. Green signal corresponds to auto-fluorescence. d, Negative control grafts of the mesendoderm part of the ventral blastopore lip onto GE did not induce Neurogenin expression. Anterior is to the left. Scale bar, 50 µm and 10 µm for section. Numbers correspond to embryos presenting similar labelling.
BMP signal
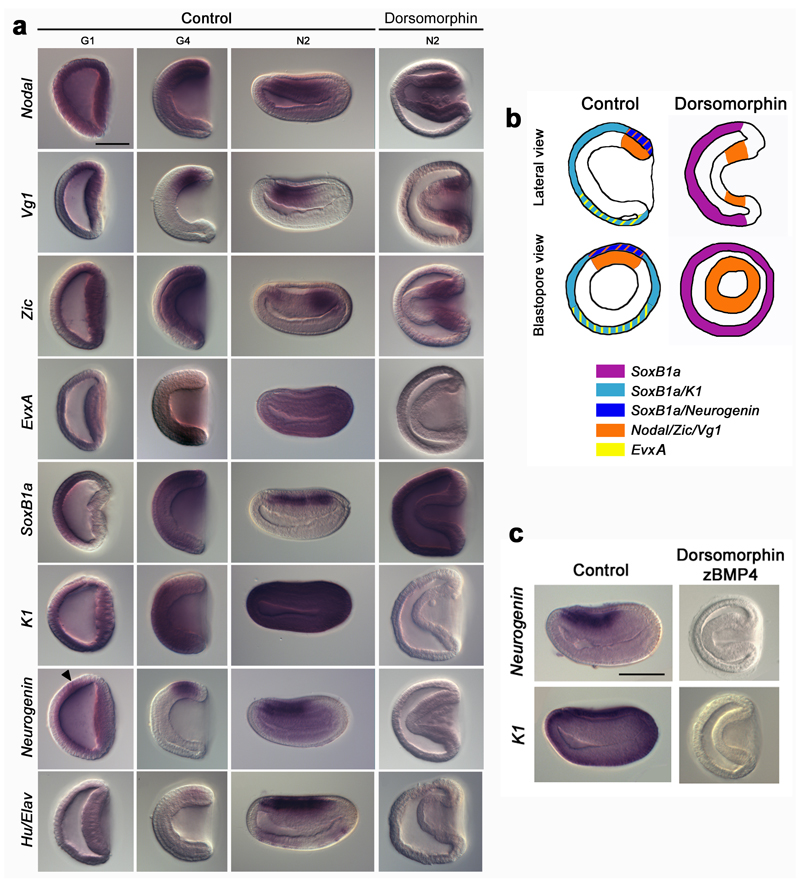
In cephalochordates, the BMP pathway is active very early during embryogenesis, as indicated by a nuclear pSmad1/5/8 signal observed before the blastula stage 18. Ectopic activation of this pathway leads to a complete ventralization of the embryo in which the whole ectoderm becomes epidermis 15, 17, whereas repression of BMP signalling via application of dorsomorphin, a chemical inhibitor of BMP receptors, at blastula stage, only induces a modest expansion of neural markers 18. Given that nuclear pSmad1/5/8 labelling was reported at cleavage stages 18, we reasoned that earlier dorsomorphin application was required to obtain more penetrant effects. When the drug was applied at cleavage stage (3.5 hpf at 19°C), embryos kept a gastrula shape, with no contact between mesendoderm and ectoderm. Dorsomorphin-treated embryos displayed a completely dorsalized mesendoderm, as revealed by the radial expression of dorsal markers (Nodal, Vg1 and Zic (Fig. 2a-b)). Strikingly, the ectoderm behaved differently. Indeed, although the expression of the ventral ectoderm marker EvxA was lost, there was no expansion of the expression of the dorsal ectoderm markers towards the ventral region (Fig 2a-b). In fact, expression of these genes was lost in the entire ectoderm, which also failed to express markers of epidermal (K1) or neural (Neurogenin, Hu/Elav) fates (Fig. 2a-b). However, the ectoderm expressed SoxB1a, ruling out a non-specific blockage of transcription. In control embryos, SoxB1a is first expressed throughout the ectoderm, until the onset of gastrulation, and is later restricted to the neural plate (Fig. 2a, Supplementary Fig. 2). Therefore, after dorsomorphin treatment, the ectoderm showed an expression profile similar to uncommitted ectoderm: SoxB1a-positive and Neurogenin/K1-negative. To verify that dorsomorphin efficiently inhibited the BMP pathway, we undertook double treatments with recombinant zBMP4 protein. In such embryos, K1 expression was not recovered, indicating that in our assays dorsomorphin totally abolished BMP activity (Fig. 2c). Altogether these data show that inhibiting the BMP signalling pathway at early developmental stages leads to dorsalization of the mesendoderm and that it blocks ectodermal cell fate commitment.
Figure 2. Role of BMP in ectodermal cell fate commitment.
a, Expression of Nodal, Vg1, Zic, EvxA, SoxB1a, K1, Neurogenin and Hu/Elav in G1, G4 and N2 stage control embryos and in dorsomorphin-treated N2 stage embryos. The white arrow head indicates the early ectodermal Neurogenin expression domain in control embryos. b, Schematic partial representation of the results presented in (a). c, Expression of K1 and Neurogenin at the N2 stage in control embryos and embryos treated with both dorsomorphin and zBMP4. All in situ hybridization images are side views with anterior towards the left. Scale bar, 50 µm.
Nodal/Activin signal and interaction with FGF and BMP
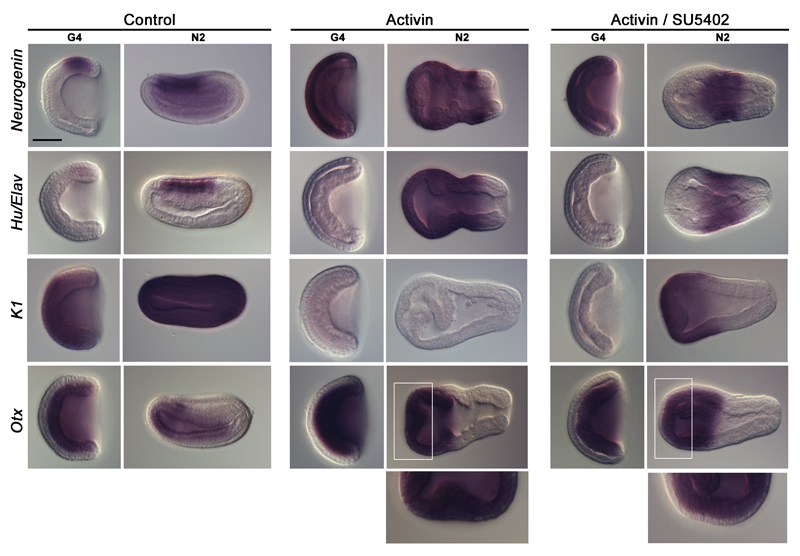
Nodal signalling is active before the blastula stage in amphioxus, as indicated by the zygotic expression of Nodal and of its target Lefty at cleavage stage22. Early ectopic activation of the Nodal/Activin signalling pathway through recombinant Activin protein treatment induces embryo dorsalization 15. In such conditions, the whole anterior ectoderm expresses Otx, whereas the whole posterior ectoderm expresses Wnt3, suggesting complete neuralization 16. On the other hand, inhibition of Nodal/Activin signalling using SB505124, an inhibitor of the Alk4/5/7 receptors, induces ventralization, and consequently the loss of neural structures 16. We confirmed that in Activin-treated embryos the ectoderm was entirely neuralized as indicated by the expression of the pan-neural markers Neurogenin and Hu/Elav, and by the complete loss of K1 expression (Fig. 3). We previously showed that embryos in which the FGF signalling pathway was inhibited still displayed ectodermal Neurogenin expression 19. We asked whether FGF signalling was involved in Nodal/Activin-mediated neuralization. Addition of the FGF receptor inhibitor SU5402 to Activin-treated embryos did not suppress Neurogenin expression at the G4 stage (Fig. 3). In contrast, at the N2 stage, neural genes expression was lost in the anterior ectoderm (Fig. 3). Conversely, K1 expression was recovered in the anterior ectoderm (Fig. 3), indicating a change of fate in the anterior ectodermal region from neural to epidermal. The Nodal/Activin signalling pathway is therefore able to completely neuralize the ectoderm independently of the FGF signal, which is however probably required for the maintenance of the anterior neural fate and/or the patterning of the anterior neural tissue.
Figure 3. Role of Nodal/Activin and FGF signalling pathways in ectoderm specification.
Expression at G4 and N2 stages of Neurogenin, Hu/Elav, K1 and Otx in control embryos, in embryos treated with recombinant Activin protein, and in embryos treated with both Activin and SU5402. All in situ hybridization images are side views with anterior towards the left. Enlargements of the anterior region (white frame) of N2 stage embryos are presented below the pictures of whole embryos to highlight Otx expression in the ectoderm. Scale bar, 50 µm.
We tested whether neuralization by Nodal/Activin pathway in amphioxus involved BMP pathway inhibition by first analyzing nuclear pSmad1/5/8. As expected, no labelling could be detected in dorsomorphin-treated neurula (Supplementary Fig. 3a). In contrast, pSmad1/5/8 nuclear staining was observed in the ectoderm of Activin-treated embryos (Supplementary Fig. 3a), suggesting that the intracellular cascade activated by BMP was not interrupted by Activin. We then analyzed the effects of dorsomorphin and Activin treatments at a global scale using an RNA-seq approach. Transcriptome analyses supported the specificity of the treatments and reinforced the previous conclusions based on in situ hybridization experiments: Activin induced neuralization, and dorsomorphin inhibited the differentiation of ectoderm cells, whereas it induced dorsalization of the mesendoderm (Supplementary Fig. 3b). We also undertook double treatments with Activin and zBMP4 recombinant proteins. Treated embryos displayed a phenotype similar to what was observed after Activin/SU5402 treatment (Supplementary Fig. 3c). The whole ectoderm expressed Neurogenin at the G4 stage but at the N2 stage this expression was lost in the anterior region (Supplementary Fig. 3c), suggesting that BMP inhibition is required for the maintenance of the anterior neural territory. Next, we tested the ability of the Nodal/Activin pathway to neuralize the ectoderm of dorsomorphin-treated embryos. Double Activin/dorsomorphin treatment often led to exogastrulation and the whole ectoderm always expressed Neurogenin (Supplementary Fig. 3c). This result demonstrates that the uncommitted ectoderm of dorsomorphin-treated embryos can resume neural differentiation. Altogether, our data strongly suggest that Nodal/Activin is able to neuralize the amphioxus ectoderm independently of BMP signalling.
Ectodermal explants and grafts
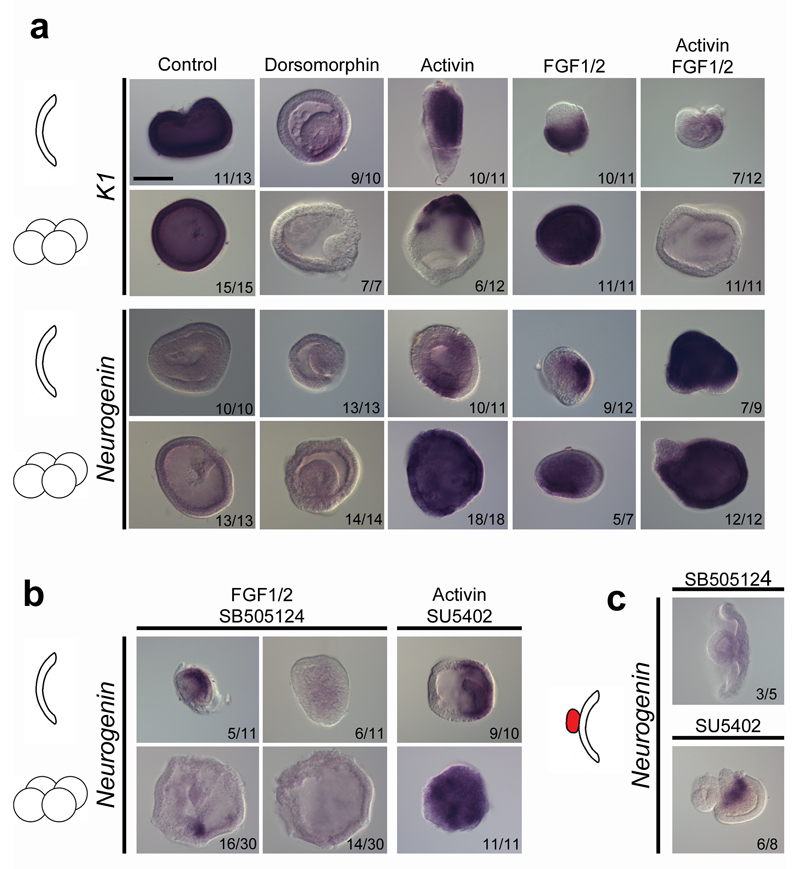
In whole embryo assays, ectodermal cells are exposed to both endogenous signals produced by the organizer and the mesendoderm, and to exogenous inducers or inhibitors. This multiplicity of signals complicates interpretations. To overcome this issue, we undertook experiments on ectodermal explants. In addition to GE, we prepared explants grown from animal blastomeres (Supplementary Fig. 4), dissected at the 8-cell stage (Blastomere Explant, BE), in order to test the effect of our reagents at an earlier stage. Similar to GE, BE ectoderm always developed into epidermis (Fig. 4a and Supplementary Fig. 5). When we inhibited the BMP signal in GE and BE, K1 expression was lost (Fig. 4a), but no expression of Neurogenin was detected (Fig. 4a), as observed in whole embryos (Fig. 2a). To test which signals could trigger neural induction in explants, we used treatments with Activin, FGF1/2, or both. In Activin and in FGF1/2 treated GE, neural identity was promoted only in a subset of cells (Fig. 4a). However, the double Activin and FGF1/2 treatment induced Neurogenin expression in a broader region, and caused nearly complete loss of K1 expression (Fig. 4a). Interestingly, these effects were more pronounced when using BE, consistent with our experiments on the time window of neural induction in whole embryo (Supplementary Fig. 6). Thus, Activin treatment alone or with FGF1/2 induced expression of Neurogenin in the whole BE and complete loss of K1 expression (Fig. 4a). These results were similar regardless of the presence or not of Brachyury-positive inner cells, showing that neuralization was independent of the presence of mesoderm. Therefore, both FGF and Nodal/Activin pathways are able to induce neural fate in ectodermal explants, probably through complementary mechanisms.
Figure 4. Nodal/Activin is the main signal triggering neural induction.
a, Expression of K1 and Neurogenin in control, dorsomorphin-treated, Activin-treated, FGF1/2-treated or Activin+FGF1/2-treated GE and BE. b, Expression of Neurogenin in FGF1/2+SB505124-treated and Activin+SU5402-treated GE and BE. c, Neurogenin expression induction by the graft of the dorsal blastoporal lip on GE is lost after SB505124 treatment in most cases, but maintained after SU5402 treatment. Number of explants showing the presented expression pattern is indicated on each panel. Scale bar, 50 µm.
In order to test putative epistatic relationships between these two signals, we undertook double treatments with Activin and SU5402 or FGF1/2 and SB505124. When treatments were applied on GE, Neurogenin was still expressed in a restricted region of the explants (Fig. 4b). In contrast, for BE, Neurogenin expression mostly disappeared after FGF1/2-SB505124 application, whereas it persisted in Activin-SU5402 treated explants (Fig. 4b). This again advocates for a primary role of Nodal/Activin and a secondary role for the FGF signalling pathway.
Finally, since we showed that the dorsal blastoporal lip was able to induce neural tissue formation in ectodermal explants (Fig. 1), we tested which could be the signal responsible for this induction. We applied FGF and Nodal/Activin signalling pathway inhibitors on grafted ectoderm explants. We found that inhibition of the FGF signal did not prevent neural tissue formation, whereas inhibition of the Nodal/Activin signal abrogated Neurogenin expression (Fig. 4c). Overall these data strongly suggest that in amphioxus the organizer triggers the first step of neuroectoderm development through the Nodal/Activin signal.
Nodal signal in Xenopus
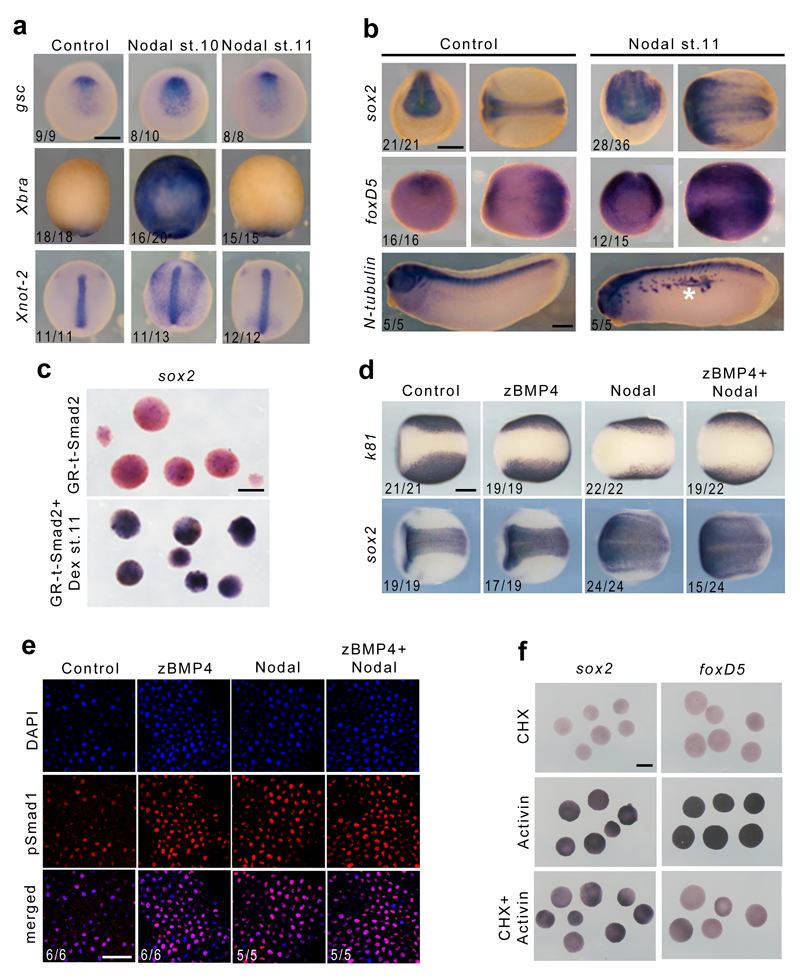
We decided to test whether in the classical vertebrate model Xenopus Nodal signalling could also induce neural tissue. As Nodal is involved in inducing the Spemann organizer 23, its role as a direct neural inducer is difficult to test. Our prior work showed that the response of Xenopus embryonic cells to Nodal signals changes with time 24. Thus, we reasoned that treatment with Nodal past the stage of competence of embryonic cells to become mesendodermal in response to this signal could allow us to evaluate its potential as a neural inducer. We found that in whole-embryos or in ectoderm explants, the application of Nodal recombinant protein at mid-gastrula stage 11 was unable to induce ectopic expression of organizer (gsc, chd) or mesendoderm markers (Xbra, Xnot-2, sox17, mixer) (Fig. 5a, Supplementary Fig. 7a). However, Nodal induced ectopic neural tissue expressing sox2 and foxD5 in both whole-embryos and in ectoderm explants (Fig. 5b and Supplementary Fig. 7a). Conversely, the epidermal marker k81 was strongly repressed (Supplementary Fig. 7a). Furthermore, direct neural induction in ectoderm explants was also achieved through the expression of an inducible form of activated Smad2 25, when induced at stage 11 (Fig. 5c and Supplementary Fig. 7b). The induced neural tissue was of anterior character as revealed by otx2 expression (Supplementary Fig. 7b), and was stable as revealed by the presence of ectopic neurons expressing N-tubulin at tailbud stage (Fig. 5b).
Figure 5. Nodal induces neural tissue in Xenopus.
a, Expression of gsc, Xbra and Xnot-2 at early neurula stage 13 in control embryos and in embryos injected with Nodal recombinant protein at stage 10 or 11. Dorsal views for gsc and Xnot-2, ventral views for Xbra. b, Expression of sox2 and foxD5 (left: front view; right: dorsal view) and N-tubulin (ectopic neurons indicated by a white asterisk) in control and Nodal-injected embryos. c, Embryos were injected with GR-t-Smad2 mRNA, animal caps were explanted at early gastrula stage, induced or not with dexamethasone at stage 11 and processed for sox2 in situ hybridization. d, Expression of sox2 and k81 in control embryos and in embryos injected with zBMP4, Nodal or both recombinant proteins. Scale bar, 250 µm. e, Confocal images of pSmad1 immunostaining and nuclear DAPI staining in control embryos and in embryos injected with zBMP4, Nodal or both recombinant proteins. Scale bar, 50 µm. f, in situ hybridization of sox2 and foxD5 in animal caps treated with cycloheximide, Activin, or both. Scale bars, 200 µm. The number of embryos showing the phenotype displayed over the total number of embryos analyzed is indicated on each panel.
We then tested whether the effect of Nodal on neural induction was achieved through inhibition or competition with the BMP signal. As previously shown 11, injection of BMP4 protein into embryos at stage 11 did not expand the epidermal tissue, whereas injection of Nodal, or co-injection of Nodal and BMP4, caused the expansion of the neural plate (Fig. 5d). Moreover, in the three conditions, we observed prominent pSmad1 nuclear staining in the ectoderm (Fig. 5e), demonstrating that Nodal does not induce neural tissue through BMP inhibition. Finally, combining cycloheximide and Activin treatments on animal caps, we showed that the early neural regulator foxD5 11, 26, but not sox2, is an immediate early target of the Nodal signalling pathway (Fig. 5f and Supplementary Fig 7c), which is consistent with the recent finding that Smad2/3 binds to an active enhancer of this gene at stage 10.5 but not at blastula stage 9 27.
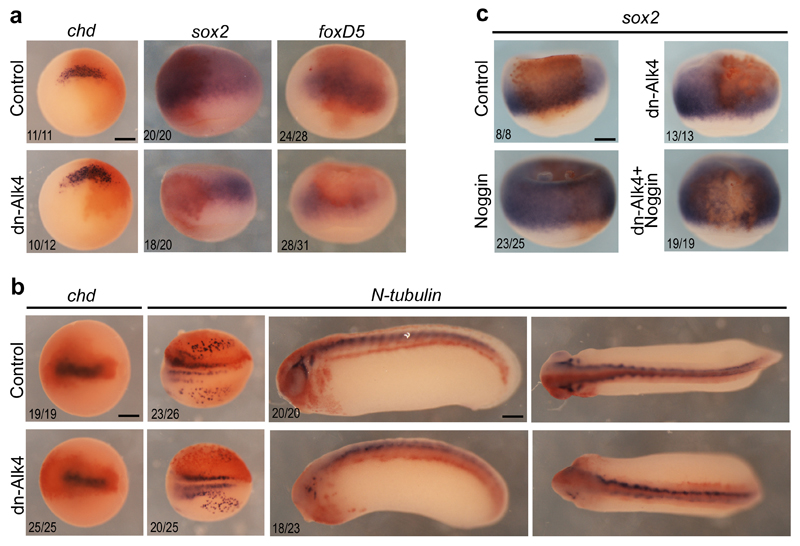
Next, we asked whether Nodal/Activin signalling was required for neural induction in Xenopus. When the inhibitors of Nodal/Activin receptors, SB505124 and SB431542, were applied on stage 11 embryos, morphogenesis proceeded normally, but neural tissue development was altered as revealed by the significant down-regulation of foxD5, sox2 and N-tubulin expression (Supplementary Fig. 8). We also injected a dominant-negative form of the Nodal/Activin receptor Alk4 (dn-Alk4) in the presumptive neural ectoderm in 8-cell embryos in order to potently inhibit the Nodal/Activin pathway from the earliest possible stage, while avoiding perturbations of the organizer mesoderm. In such embryos, chd expression was maintained, whereas foxD5, sox2 and N-tubulin expression was severely down-regulated in injected ectodermal cells (Fig. 6a, b). Strikingly, Noggin recombinant protein injection could neuralize control ectoderm cells but not cells that received dn-Alk4, further demonstrating the BMP-independent role of the Nodal/Activin pathway (Fig. 6c). We conclude that in standard assays in Xenopus, Nodal behaves as a bona fide neural inducer, as it is capable of directly activating neural markers in the absence of organizer mesoderm, is required for neural tissue development and functions without interfering with the BMP signal.
Figure 6. Activin/Nodal signaling is required within the ectoderm for neural induction in Xenopus.
a, Expression of chd, sox2 and foxD5 at stage 10 in control embryos and in embryos injected with dnAlk4 mRNA in dorsal animal cells. Vegetal view for chd and dorsal view for sox2 and foxD5. b, Expression of chd at stage 13 and N-tubulin at stage 15 and 25 in control embryos and in embryos injected with dnAlk4 mRNA in dorsal animal cells. Dorsal view for chd and N-tubulin at stage 15 and stage 25 (left) and lateral view for N-tubulin at stage 25 (right). c, Expression of sox2 at stage 10 in control embryos and in embryos injected with dnAlk4 mRNA in dorsal animal cells. Noggin recombinant protein was injected in the blastocoele at stage 8 to induce neural tissue. Embryos are shown in dorsal view. In all cases, embryos were injected with fixable fluorescein lysine dextran and revealed by immunostaining (orange). The number of embryos showing the phenotype displayed over the total number of embryos analyzed is indicated on each panel. Scale bars, 250µm.
Discussion
Although previous embryological studies 14 and gene expression data 15 suggested that the dorsal blastopore lip of amphioxus has the same properties as the vertebrate organizer, no direct evidence for its capacity to induce neural fate was ever reported. By using challenging microsurgery techniques we demonstrate that the amphioxus dorsal blastopore lip is indeed equivalent to the vertebrate dorsal organizer. It can induce a secondary nervous system when grafted into a host embryo and convert ectodermal cells from epidermal to neural identity.
The results we present in this work also suggest that the default model does not account for neural induction in amphioxus. Although BMP inhibition is required for neural tissue to emerge, it is not sufficient, and instructive cues must be delivered. Indeed, both in whole embryos and in explants, inhibition of BMP signalling led to the maintenance of an undifferentiated state of the ectoderm. At first glance, this result appears different from what was published when the same approach was undertaken at later stages on whole embryos 18. Indeed, Kozmikova and co-workers observed a modest lateral expansion of the specific neural plate markers Brn1/2/4 and SoxB1c, whereas SoxB1a expression became widespread in the non-neural ectoderm. The effects caused by BMP inhibition in their study are in fact consistent with our own observations using earlier treatment. We suggest, however, that late BMP inhibition probably did not fully prevent neural induction in this assay and that the enlarged expression of the neural markers Brn1/2/4 and SoxB1c would reflect the conversion of the neural plate border territory into axial neural ectoderm, although this hypothesis remains to be tested.
We also provide compelling evidence that both Nodal/Activin and FGF signals are implicated in different steps of CNS formation in amphioxus. Altogether, our data suggest that Nodal/Activin is the main neural inducing signal emanating from the organizer, whereas FGF would be implicated in the maintenance/patterning of the anterior neural territory. Importantly, we also show that the activity of Nodal/Activin on neural induction is independent of the presence of mesodermal cells. Likewise, we report here that neuralization of ectoderm by Nodal in Xenopus can occur independently of the presence of organizer mesoderm. Moreover, neuralization by Nodal/Activin is maintained in the presence of active BMP signalling in both amphioxus and Xenopus.
Our results have major implications for the evolution of the molecular control of neural induction. First, we propose that the default model does not account for neural induction throughout the chordate lineage. Indeed, although BMP signal inhibition is necessary for neural induction in all vertebrates 3, BMP plays no role in this process in tunicates 12 and BMP inhibition appears insufficient to trigger neural induction in chick and amphioxus although it may be required to maintain the anterior neural territory in both species 9. The situation is different in the urochordate Ciona, where FGF is the main neural inducer13, whereas Nodal is required for posterior neural tube formation and for the specification of trunk epidermal sensory neurons 28–30. Concerning vertebrates, Nodal activity was shown to be required to prevent precocious neural differentiation in the mouse pluripotent epiblast 31. Likewise, concomitant inhibition of Smad1 and Smad2 was proposed as a necessary condition for neural induction in Xenopus 32. In contrast, the activity of Smad2/3 seems to be required for neural induction in zebrafish 33, raising the possibility that multiple phases of intervention of this pathway may complicate interpretations. Here, we provide new evidence showing that Nodal behaves as a bona fide neural inducer in Xenopus. Key to this demonstration was our finding that the mesendoderm and neural inductive capacities of Nodal could be uncoupled. Thus, our assay provides a new and valuable technical framework to work out the mechanisms of neural induction in this animal. Multiple Nodal ligands are expressed in the Spemann organizer, possibly contributing directly to neural induction 24, 34. In support to this idea, we showed here that in two assays designed to preserve organizer formation, Nodal pathway inhibition caused severe loss of neural marker gene expression. Collectively, our data suggest that the implication of Nodal/Activin signalling in the first step of CNS formation might be ancestral in chordates. Interestingly, it has been recently shown that Nodal is also able to trigger secondary body axis formation and neural fate commitment in the sea urchin 35, pushing back the possible ancestral role of this signal in neural induction to the base of the deuterostome lineage. In conclusion, we suggest that neural induction can be triggered by BMP inhibition, by FGF and by Nodal signalling but that the precise spatio-temporal contribution of each pathway may vary across chordate lineages.
Materials and Methods
Obtaining embryos, micro-dissection and graft experiments
Branchiostoma lanceolatum adults were collected at the Racou beach in Argelès-sur-Mer (France). Spawning was induced as previously described 36, 37. Staging is according to Hirakow and Kajita 38, 39. For explantation experiments, embryos were fertilized and kept in scratched Petri dishes. Gastrula stage explants (GE) preparation was undertaken on embryos that were not previously dechorionated except when GE were prepared for graft experiments. To maintain the chorion surrounding the embryo immobile, the bottom of the Petri dish was cut with a micro-scalpel to create two or three stripes. When the chorion was immobilized, the position of the embryo inside the chorion was stabilized (with the antero-posterior axis parallel to the bottom of the Petri dish) and GE explants were obtained using dissection perpendicular to the Petri dish with a micro-scalpel. For blastomere explants (BE) preparation and grafting, the embryos were manually dechorionated just before the first cell division by projection against the borders of an agarose coated Petri dish (0,8% agarose in 0.2 µm filtered sea water) until micro-dissection. Embryos were dissected with an eyelash. For grafts experiments, after micro-dissection, hosts and grafts were gently put into contact and were left stationary for one hour, the time needed for at least one cell division to occur. To avoid any effect of the agarose coat on the molecules used for treatments, all the treatments were undertaken in scratched Petri dishes. For graft experiments using fluorescent embryos, Dextran (10,000 MW) coupled to Texas Red (Invitrogen) was injected in unfertilized eggs. Eggs obtained from NASCO Xenopus laevis females were fertilized in vitro, de-jellied, cultured, staged, and injected as previously described 10, 40.
Embryo and explant treatments
Amphioxus embryos, explants or grafts were treated with the following molecules: dorsomorphin (35 µM for whole embryos and 10 µM for explants, prepared as in 41, Sigma), Activin (50 ng/mL, human recombinant protein, R&D), zBMP4 (250 ng/mL, zebrafish recombinant protein, R&D ), SB505124 (50 µM, dissolved in dimethyl sulfoxide (DMSO), Sigma), FGF1/2 (2 µg/mL, B. lanceolatum recombinant protein), SU5402 (50 µM, dissolved in DMSO, Calbiochem). The concentration used were defined after pilot experiments using the following ranges of drug or recombinant protein concentration: dorsomorphin: 10 µM-50 µM, Activin: 10 ng/mL-100 ng/mL, zBMP4: 50-250 ng/mL, SB505124: 25 µM-100µM, FGF1/2: 500 ng/mL-2 µg/mL, SU5402: 25 µM-100µM. Whole embryos were treated at 3.5 hpf at 19°C except when specified. Explants and grafted explants/embryos were directly treated following the explantation or graft procedure. For time window experiments, embryos were washed at least 4 times after treatment to ensure that no drug/recombinant protein were left in the culture medium. Xenopus embryos were injected in the blastocoel with 10 ng mouse recombinant Nodal protein (R&D), 3.5 ng zebrafish recombinant BMP4 protein (R&D), or 30 ng recombinant human Noggin (R&D). dn-Alk4 and GR-t-Smad2 25 mRNAs were synthesized with the Ambion mMessage mMachine kit. 8-cell embryos were injected with 3 ng of dnAlk4 in one of the two dorsal-animal blastomere. Fixable fluorescein lysine dextran (FLDx, 2.5 ng/cell) was co-injected to sort properly injected embryos, and anti-fluorescein immunodetection (anti-fluorescein/alkaline phosphatase antibody and iodonitrotetrazolium/5-bromo-4-chloro-3-indolyl phosphate substrate, Roche) was performed to reveal injected territories in fixed embryos. 2-cell embryos were injected with 400 pg of GR-t-Smad2 mRNA, animal caps were explanted at early gastrula stage 10 and treated with dexamethasone at 10 µg/ml. Negative controls were treated with 1% ethanol. Cycloheximide treatment (10 μg/ml) was started 1 hour prior to the addition of Activin (5 ng/ml, human recombinant protein, R&D) to avoid any delay of action, and treatment was continued for 2 hours at 18°C. SB505124 (Sigma) and SB431542 (Sigma) were dissolved in DMSO, diluted 1/100 in 0,1X MBS (final concentrations 200µM and 800µM, respectively) and applied on gastrula stage 11 embryos. Control embryos were treated with 1% DMSO (Sigma) diluted in 0,1X MBS.
In situ hybridization, immunostaining and quantitative RT-PCR
Amphioxus in situ hybridization was performed as previously described 42 and unless mentioned otherwise were undertaken on at least 12 embryos, all of them showing the same phenotype. Amphioxus anti-tubulin immunostaining was undertaken as described in 19 using a primary antibody against acetylated tubulin (Sigma T6793, 1:500), and a secondary antibody (1:500) coupled to fluorescein. Immunostaining against pSmad1/5/8 was undertaken using a rabbit polyclonal anti-pSmad1/5/8 primary antibody (Cell Signaling 9511L, 1:150) and a secondary antibody (1:500) coupled to FITC. Photographs were processed in ImageJ using the Parallel Iterative Deconvolution 2D plug-in. Xenopus embryos or animal caps were processed for in situ hybridization as described in 11. For quantitative RT-PCR, total RNAs were extracted with the RNeasy mini kit (Qiagen), cDNAs were synthesized using the SuperScript II reverse transcriptase (Invitrogen), and amplifications were performed in the presence of SYBR Green mix (Invitrogen) on an iQ5 machine (Bio-Rad). Immunostaining against pSmad1 in Xenopus was undertaken as described in 43 using a rabbit polyclonal anti-pSmad1/5/8 primary antibody (Cell Signaling 9511L, 1:100) and a secondary antibody (1:500) coupled to Alexa561. Accession numbers and primer sequences are given in Supplementary Tables 1 and 2.
RNA-seq analysis
Embryos from two females were separated in three independent batches for each female: control, dorsomorphin-treated and Activin-treated. Embryos were collected from each batch at the G4 (half of the embryos) and N2 stages and frozen in liquid nitrogen. RNA extraction was performed using the RNeasy mini kit (Qiagen). Sequencing was done at the CRG Genomics Unit and raw reads submitted to the BioProject database (accession number PRJNA354850). The clean sequencing reads from each sample were mapped to a Branchiostoma lanceolatum transcriptome assembly 44 using the software Bowtie2 with default parameters 45. To calculate and normalize the mapped read counts, we used the software RSEM (http://deweylab.biostat.wisc.edu/rsem/) 46. We analyzed the differential expression between control and treated embryos for selected genes and generated a heatmap using Morpheus (https://software.broadinstitute.org/morpheus/).
Data availability statement
Accession numbers of sequences used for in situ hybridization probe synthesis and the sequences of primers used for quantitative PCR analysis are given in Supplementary Tables 1 and 2. RNA-seq raw reads are available at the BioProject database (accession number PRJNA354850).
Statement that all experiments were performed in accordance with relevant guidelines and regulations
All the experiments were performed following the Directive 2010/63/EU of the European parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. All Xenopus experiments were approved by the "Direction départementale de la Protection des Populations, Pôle Alimentation, Santé Animale, Environnement, des Bouches du Rhône" (agreement number F 13 055 21). Ripe adults from the Mediterranean invertebrate amphioxus species (Branchiostoma lanceolatum) were collected at the Racou beach near Argelès-sur-Mer, France, (latitude 42° 32’ 53” N and longitude 3° 03’ 27” E) with a specific permission delivered by the Prefect of Region Provence Alpes Côte d’Azur.
Supplementary Material
Acknowledgements
The laboratory of H.E. was supported by the CNRS and the ANR-16-CE12-0008-01 and S.B. by the Institut Universitaire de France. This project was supported in L.K.’s laboratory by ANR BSV2-021-02 and by Fondation ARC. M.I. is supported by an ERC Starting Grant (grant agreement No ERC-StG-LS2-637591) and the Spanish Ministry of Economy and Competitiveness (‘Centro de Excelencia Severo Ochoa 2013-2017’, SEV-2012-0208 to the CRG). Some of the Xenopus experiments were performed on PiCSL-FBI core facility (IBDM, AMU-Marseille), member of the France-BioImaging national research infrastructure. Some of the amphioxus experiments were undertaken on the Cytometry and Imaging Platform of the Observatoire Océanologique de Banyuls-sur-mer. RNA sequencing was performed at the CRG Genomics facility. We thank Sébastien Darras for technical help and Mohamed Belgacem for amphioxus FGF1/2 in vitro production.
Footnotes
Author contributions statement
Conceptualization , Y.L.P., L.K., H.E. and S.B.; Investigation, Y.L.P., G.L., P.S., M.C., A. L., L. S., M. I., H.E. and S.B.; Writing – Original Draft , Y.L.P., L.K., H.E. and S.B.; Funding Acquisition , M. I., L.K., H.E. and S.B.; Supervision , L.K., H.E. and S.B.
Competing Financial Interests statement
The authors declare no competing financial interests.
References
- 1.Spemann H, Mangold H. In: Fundation of Experimental Embryology. Willer BH, Oppenheimer JM, editors; Hamburger V, translator. New York: 1924. pp. 144–184. [Google Scholar]
- 2.Hemmati-Brivanlou A, Melton D. Vertebrate embryonic cells will become nerve cells unless told otherwise. Cell. 1997;88:13–7. doi: 10.1016/s0092-8674(00)81853-x. [DOI] [PubMed] [Google Scholar]
- 3.Stern CD. Neural induction: 10 years on since the 'default model'. Curr Opin Cell Biol. 2006;18:692–7. doi: 10.1016/j.ceb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 4.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozair MZ, Kintner C, Brivanlou AH. Neural induction and early patterning in vertebrates. Wiley Interdiscip Rev Dev Biol. 2013;2:479–98. doi: 10.1002/wdev.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–8. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- 7.Wilson SI, Graziano E, Harland R, Jessell TM, Edlund T. An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol. 2000;10:421–9. doi: 10.1016/s0960-9822(00)00431-0. [DOI] [PubMed] [Google Scholar]
- 8.Launay C, Fromentoux V, Shi DL, Boucaut JC. A truncated FGF receptor blocks neural induction by endogenous Xenopus inducers. Development. 1996;122:869–80. doi: 10.1242/dev.122.3.869. [DOI] [PubMed] [Google Scholar]
- 9.Linker C, Stern CD. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development. 2004;131:5671–5681. doi: 10.1242/dev.01445. [DOI] [PubMed] [Google Scholar]
- 10.Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. Epub 2004 Dec 08. [DOI] [PubMed] [Google Scholar]
- 11.Marchal L, Luxardi G, Thome V, Kodjabachian L. BMP inhibition initiates neural induction via FGF signaling and Zic genes. Proc Natl Acad Sci U S A. 2009;106:17437–42. doi: 10.1073/pnas.0906352106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darras S, Nishida H. The BMP/CHORDIN antagonism controls sensory pigment cell specification and differentiation in the ascidian embryo. Dev Biol. 2001;236:271–88. doi: 10.1006/dbio.2001.0339. [DOI] [PubMed] [Google Scholar]
- 13.Bertrand V, Hudson C, Caillol D, Popovici C, Lemaire P. Neural tissue in ascidian embryos is induced by FGF9/16/20, acting via a combination of maternal GATA and Ets transcription factors. Cell. 2003;115:615–27. doi: 10.1016/s0092-8674(03)00928-0. [DOI] [PubMed] [Google Scholar]
- 14.Tung TC, Wu SC, Tung YYF. Experimental studies on the neural induction in amphioxus. Scientia Sinica. 1962;XI:805–820. [Google Scholar]
- 15.Yu JK, et al. Axial patterning in cephalochordates and the evolution of the organizer. Nature. 2007;445:613–7. doi: 10.1038/nature05472. [DOI] [PubMed] [Google Scholar]
- 16.Onai T, Yu JK, Blitz IL, Cho KW, Holland LZ. Opposing Nodal/Vg1 and BMP signals mediate axial patterning in embryos of the basal chordate amphioxus. Dev Biol. 2010;344:377–89. doi: 10.1016/j.ydbio.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu JK, Meulemans D, McKeown SJ, Bronner-Fraser M. Insights from the amphioxus genome on the origin of vertebrate neural crest. Genome Res. 2008;18:1127–32. doi: 10.1101/gr.076208.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozmikova I, Candiani S, Fabian P, Gurska D, Kozmik Z. Essential role of Bmp signaling and its positive feedback loop in the early cell fate evolution of chordates. Dev Biol. 2013;382:538–54. doi: 10.1016/j.ydbio.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Bertrand S, et al. Amphioxus FGF signaling predicts the acquisition of vertebrate morphological traits. Proc Natl Acad Sci U S A. 2011;108:9160–5. doi: 10.1073/pnas.1014235108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weng W, Stemple DL. Nodal signaling and vertebrate germ layer formation. Birth Defects Res C Embryo Today. 2003;69:325–32. doi: 10.1002/bdrc.10027. [DOI] [PubMed] [Google Scholar]
- 21.Tung TC, Wu SC, Tung YF. The development of isolated blastomeres of Amphioxus. Sci Sin. 1958;7:1280–320. [PubMed] [Google Scholar]
- 22.Morov AR, Ukizintambara T, Sabirov RM, Yasui K. Acquisition of the dorsal structures in chordate amphioxus. Open Biology. 2016;6 doi: 10.1098/rsob.160062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–83. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luxardi G, Marchal L, Thome V, Kodjabachian L. Distinct Xenopus Nodal ligands sequentially induce mesendoderm and control gastrulation movements in parallel to the Wnt/PCP pathway. Development. 2010;137:417–26. doi: 10.1242/dev.039735. [DOI] [PubMed] [Google Scholar]
- 25.Vonica A, Brivanlou AH. The left-right axis is regulated by the interplay of Coco, Xnr1 and derriere in Xenopus embryos. Dev Biol. 2007;303:281–94. doi: 10.1016/j.ydbio.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 26.Yan B, Neilson KM, Moody SA. foxD5 plays a critical upstream role in regulating neural ectodermal fate and the onset of neural differentiation. Dev Biol. 2009;329:80–95. doi: 10.1016/j.ydbio.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta R, Wills A, Ucar D, Baker J. Developmental enhancers are marked independently of zygotic Nodal signals in Xenopus. Dev Biol. 2014;395:38–49. doi: 10.1016/j.ydbio.2014.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roure A, Lemaire P, Darras S. An otx/nodal regulatory signature for posterior neural development in ascidians. PLoS Genet. 2014;10:e1004548. doi: 10.1371/journal.pgen.1004548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mita K, Fujiwara S. Nodal regulates neural tube formation in the Ciona intestinalis embryo. Dev Genes Evol. 2007;217:593–601. doi: 10.1007/s00427-007-0168-x. [DOI] [PubMed] [Google Scholar]
- 30.Ohtsuka Y, Matsumoto J, Katsuyama Y, Okamura Y. Nodal signaling regulates specification of ascidian peripheral neurons through control of the BMP signal. Development. 2014;141:3889–99. doi: 10.1242/dev.110213. [DOI] [PubMed] [Google Scholar]
- 31.Camus A, Perea-Gomez A, Moreau A, Collignon J. Absence of Nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev Biol. 2006;295:743–55. doi: 10.1016/j.ydbio.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 32.Chang C, Harland RM. Neural induction requires continued suppression of both Smad1 and Smad2 signals during gastrulation. Development. 2007;134:3861–72. doi: 10.1242/dev.007179. [DOI] [PubMed] [Google Scholar]
- 33.Jia S, Wu D, Xing C, Meng A. Smad2/3 activities are required for induction and patterning of the neuroectoderm in zebrafish. Dev Biol. 2009;333:273–84. doi: 10.1016/j.ydbio.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 34.Joseph EM, Melton DA. Xnr4: a Xenopus nodal-related gene expressed in the Spemann organizer. Dev Biol. 1997;184:367–72. doi: 10.1006/dbio.1997.8510. [DOI] [PubMed] [Google Scholar]
- 35.Lapraz F, Haillot E, Lepage T. A deuterostome origin of the Spemann organiser suggested by Nodal and ADMPs functions in Echinoderms. Nat Commun. 2015;6:8434. doi: 10.1038/ncomms9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuentes M, et al. Insights into spawning behavior and development of the european amphioxus (Branchiostoma lanceolatum) J Exp Zoolog B Mol Dev Evol. 2007;308:484–93. doi: 10.1002/jez.b.21179. [DOI] [PubMed] [Google Scholar]
- 37.Fuentes M, et al. Preliminary observations on the spawning conditions of the European amphioxus (Branchiostoma lanceolatum) in captivity. J Exp Zoolog Part B Mol Dev Evol. 2004;302:384–91. doi: 10.1002/jez.b.20025. [DOI] [PubMed] [Google Scholar]
- 38.Hirakow R, Kajita N. Electron microscopic study of the development of Amphioxus, Branchiostoma belcheri tsingtauense: The Neurula and Larva. Acta Anat Nippon. 1994;69:1–13. [PubMed] [Google Scholar]
- 39.Hirakow R, Kajita N. Electron microscopic study of the development of amphioxus, Branchiostoma belcheri tsingtauense: The gastrula. J Morphology. 1991;207:37–52. doi: 10.1002/jmor.1052070106. [DOI] [PubMed] [Google Scholar]
- 40.Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) North Holland, Amsterdam: 1994. [Google Scholar]
- 41.Dolez M, Nicolas JF, Hirsinger E. Laminins, via heparan sulfate proteoglycans, participate in zebrafish myotome morphogenesis by modulating the pattern of Bmp responsiveness. Development. 2011;138:97–106. doi: 10.1242/dev.053975. [DOI] [PubMed] [Google Scholar]
- 42.Somorjai I, Bertrand S, Camasses A, Haguenauer A, Escriva H. Evidence for stasis and not genetic piracy in developmental expression patterns of Branchiostoma lanceolatum and Branchiostoma floridae, two amphioxus species that have evolved independently over the course of 200 Myr. Dev Genes Evol. 2008;218:703–13. doi: 10.1007/s00427-008-0256-6. [DOI] [PubMed] [Google Scholar]
- 43.Plouhinec JL, Zakin L, Moriyama Y, De Robertis EM. Chordin forms a self-organizing morphogen gradient in the extracellular space between ectoderm and mesoderm in the Xenopus embryo. Proc Natl Acad Sci U S A. 2013;110:20372–9. doi: 10.1073/pnas.1319745110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oulion S, Bertrand S, Belgacem MR, Le Petillon Y, Escriva H. Sequencing and analysis of the Mediterranean amphioxus (Branchiostoma lanceolatum) transcriptome. PLoS One. 2012;7:e36554. doi: 10.1371/journal.pone.0036554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Accession numbers of sequences used for in situ hybridization probe synthesis and the sequences of primers used for quantitative PCR analysis are given in Supplementary Tables 1 and 2. RNA-seq raw reads are available at the BioProject database (accession number PRJNA354850).