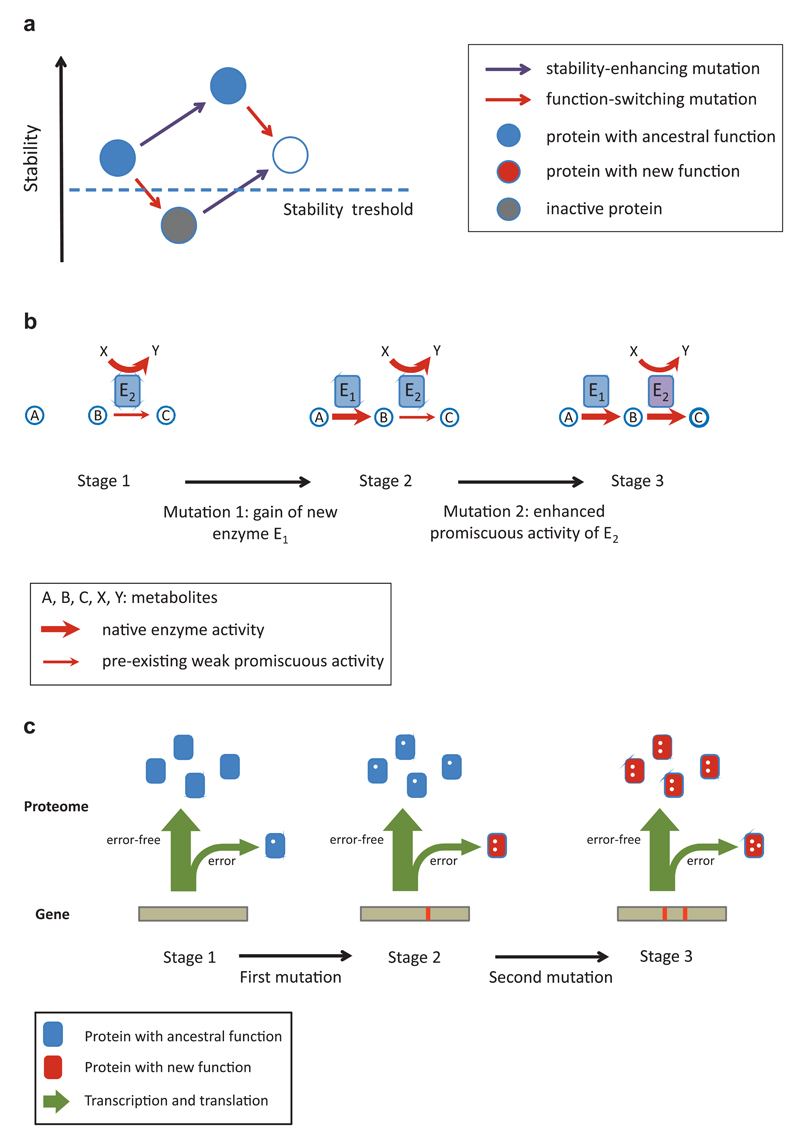
Figure 4. Molecular springboards of complex adaptations.
Molecular mechanisms that potentiate the establishment of complex adaptations by eliminating fitness valleys (A) or opening up more direct mutational paths (B-C). A) Permissive mutations that increase protein stability allow the fixation of function-altering mutations that would otherwise inactivate the protein. Note that the stability-enhancing mutation might not have any fitness effect. B) The presence of low-level enzyme side activities facilitates the adaptive evolution of multi-step metabolic pathways. A two-step pathway with metabolites A-C is depicted where the second metabolic step can be weakly catalyzed by the promiscuous side activity of enzyme E2 (stage 1). Note that E2 has a primary enzymatic activity outside of the pathway of interest. Gain of an enzyme (E1) catalyzing the first reaction immediately confers a fitness advantage as it allows the operation of the pathway, albeit with low activity (stage 2). A second mutation enhances the side activity of E2 and thereby results in a fully functional pathway (stage 3). C) Phenotypic mutations allow selection for intermediate mutations that would otherwise be neutral. The figure depicts a situation where two mutations are required for a novel protein function. Owing to transcriptional and translational errors, a small fraction of the proteome already possesses one of the mutations in a non-heritable form (stage 1). A genotype carrying the other mutation thus has a selective advantage as some of its proteins will carry out the new function (stage 2). A later adaptive genetic mutation provides the full fitness benefit by converting all protein molecules within the cell from the ancestral into the new function (stage 3). Mutations / errors are depicted by white dots.