Fig. 4. Bimodal activation of TRPV1 by BmP01 and proton.
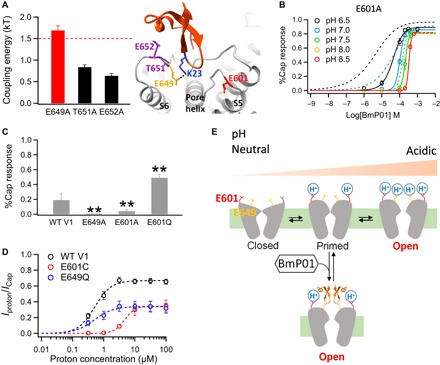
(A) A salt bridge mediates BmP01-TRPV1 interaction. Left: Comparison of coupling energy between K23 of BmP01 and the pre-S6 channel residues (n = 4 to 7) with the 1.5-kT threshold for direct interaction indicated with a dashed line. Right: Structural model of the BmP01-TRPV1 complex, with the two proton-binding sites (E601 and E649) highlighted in red and orange, respectively. (B) Concentration-response relationships of E601A at varying pH levels fitted to a Hill equation (n = 5 to 10). Concentration-response relationships of WT hTRPV1 at the same pH levels (Fig. 2D) are superimposed as dashed lines for comparison. (C) Comparison of responses by WT hTRPV1 and its point mutants to 100 μM BmP01 at neutral acidity (pH 7.5) (n = 3 to 5). **P < 0.01. (D) Proton concentration-response relationships of WT mTRPV1, E601Q, and E649Q mutants fitted to a Hill equation (n = 4 to 7). (E) Cartoon summarizing the bimodal activation of TRPV1 by proton and BmP01. At pH 6.5, proton binding to the high-affinity site E601 potentiates TRPV1; under this situation, BmP01 binding to E649 leads to strong activation. At even lower pH levels, protons bind to both E601 and E649 to open the channel without BmP01.
