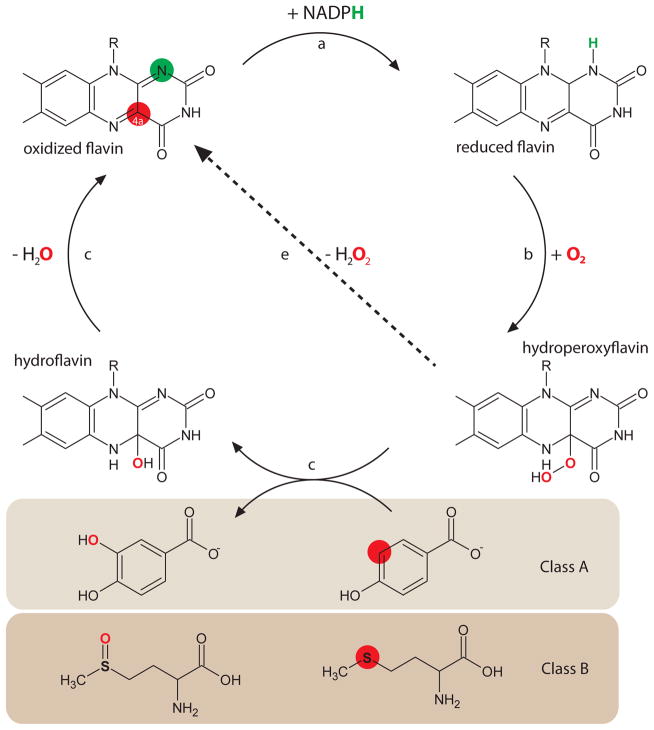
Figure 2. Reaction mechanism of flavin-dependent monooxygenases.
Oxidized FAD (represented here by its isoalloxaxine ring) reacts with NAPDH forming the neutral hydroquinone (a). Molecular oxygen reacts at position C4a (red spot) forming the hydroperoxyflavin (b), that can oxidize/hydroxilate substrates (c and d) or decompose releasing H2O2 (e, “uncoupled” reaction). Class A FMOs are considered hydroxylases, as depicted here in the reaction of hydroxylation of para-hydroxybenzoate. Class B FMOs may oxidize thioether, as shown in the lower part with methionine as substrate. Adapted from67,60.