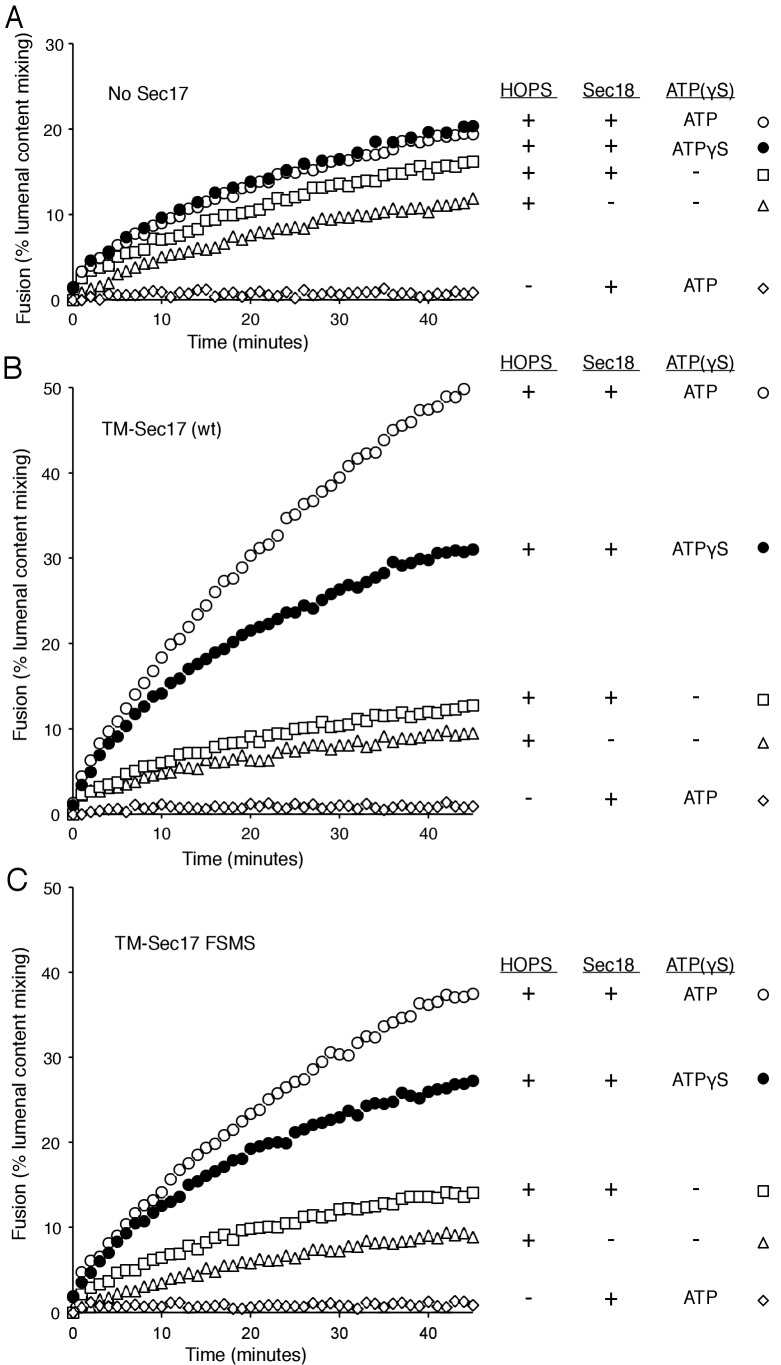
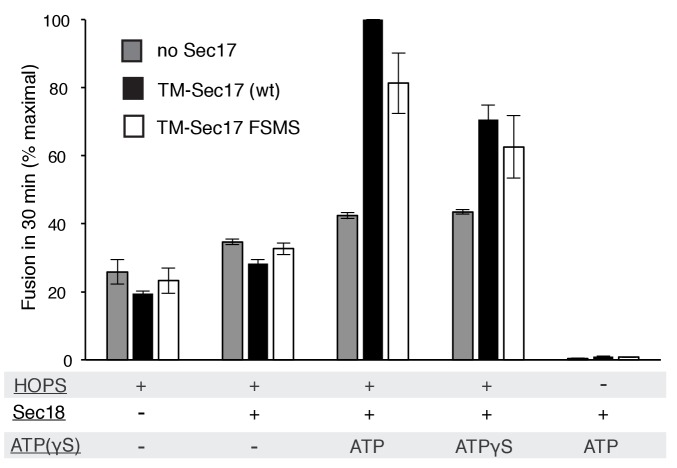
Figure 4. Membrane-anchoring Sec17 bypasses the requirement for the apolarity of its N-domain loop.
The transmembrane domain from the Qb-SNARE Vti1 was joined to the N-terminus of Sec17 (wt) or Sec17 F21S,M22S (FSMS) to give TM-Sec17 (wt) and TM-Sec17 FSMS. (A) No Sec17, (B) TM-Sec17 (wt), or C). TM-Sec17 FSMS were included at a 1:8000 molar ratio to lipids in the reconstitution of Ypt7(GTP):R-SNARE and Ypt7(GTP):QaQb-SNARE proteoliposomes (Ypt7 and each SNARE were added at 1:8000 and 1:40,000 molar ratios to lipids, respectively). Fusion incubations with these proteoliposomes had HOPS, Qc, Sec18, and no ATP, ATP, or ATPγS as indicated.
DOI: http://dx.doi.org/10.7554/eLife.26646.013