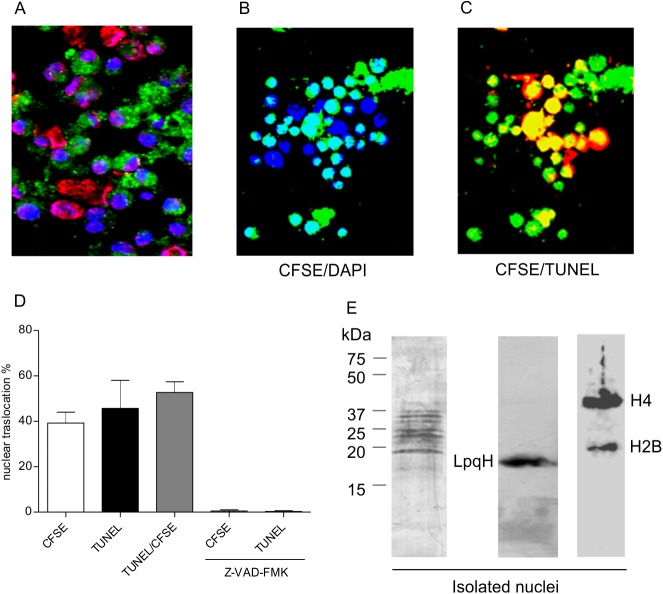
Fig 2. Mycobacterial antigens are translocated from the cytosol into the nuclei of apoptotic cells.
To examine the intracellular movement of proteins that induce apoptosis, MØs were incubated with CFSE-labeled MsmegLpqH cell wall proteins. (A) After 1 h, MØ nuclei were stained with DAPI and TUNEL. Confocal immunofluorescence images show cytoplasmic vacuoles containing phagocytosed mycobacterial proteins in most of the cells (original magnification, 40x). (B, D) In addition, 39.9% of the cells exhibited overlapping DAPI (excitation 359 nm, emission 461 nm) and CFSE fluorescence (excitation 493 nm, emission 525 nm) (original magnification 40x). (C, D) Thus, translocation of mycobacterial proteins from cytosolic deposits to the nuclei was observed; 45.7% of the nuclei were apoptotic as shown by TUNEL, and 52.6% of the apoptotic nuclei exhibited overlapping TUNEL and CFSE fluorescence (original magnification 40x). (D) Nuclear translocation of proteins and apoptosis were virtually eliminated when the MØs were pretreated with the pancaspase inhibitor, Z-VAD-FMK. To identify the mycobacterial proteins that translocated to the nucleus, MØ were incubated with MsmegLpqH cell wall proteins for 1 h. (E) Nuclear extracts of these cells were then subjected to immunoblotting with a rabbit anti-M smegmatis antiserum and a secondary HRP labeled anti-rabbit IgG antibody. Several antigenic bands were observed that ranged in size from 20 kDa to 37 kDa. Staining with a mAb confirmed the nuclear translocation of LpqH and bands with sizes of 37 kDa and 20 kDa corresponding to histones H4 and H2B. The results shown are representative of three independent experiments.