Figure 4. P-TEFb-dependent ADP-ribosylation of NELF by PARP-1.
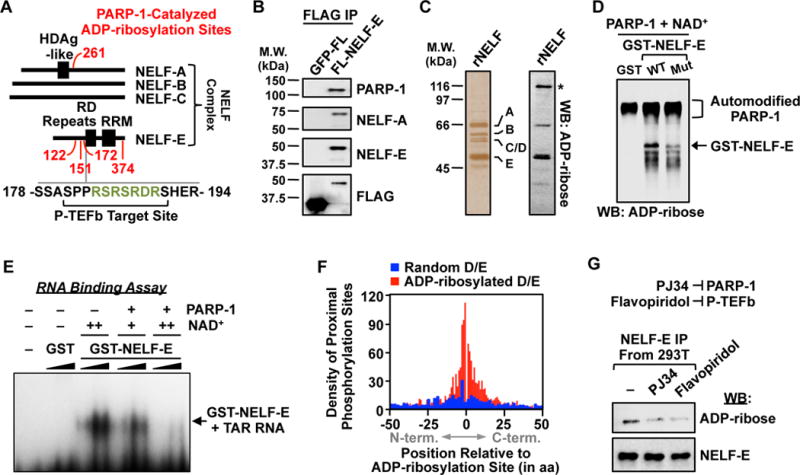
(A) Schematic showing the distribution of PARP-1 ADP-ribosylation sites (red), P-TEFb phosphorylation sites, and a PARP target-enriched 7-mer RSRSRDR (green) on proteins in the NELF complex.
(B) Western blot analysis of immunoprecipitated FLAG-tagged NELF-E or GFP from 293T cells.
(C) Silver stained SDS-PAGE gel (left) and ADP-ribose Western blot (right) of immunopurified NELF complex. Asterisk = automodified PARP-1.
(D) Western blot for ADP-ribose of in vitro modification reactions containing GST, GST-tagged wild-type NELF-E, or GST-tagged ADP-ribosylation site point mutant NELF-E, PARP-1, and NAD+ as indicated.
(E) NELF-E/TAR RNA electrophoretic mobility shift assay with or without PARP-1-mediated ADP-ribosylation. GST or GST-NELF-E was titrated between 0.1 to 1.0 μM and NAD+ was added at 25 μM (+) or 100 μM (++) during the ADP-ribosylation reaction.
(F) Histogram of the relationship between ADP-ribosylation sites identified herein and the nearest incidence of known phosphorylation modifications on PARP target proteins.
(G) Western blot analysis of immunoprecipitated FLAG-tagged NELF-E from 293T cells treated with vehicle, the PARP inhibitor PJ34, or the P-TEFb/CDK9 inhibitor flavopiridol.
