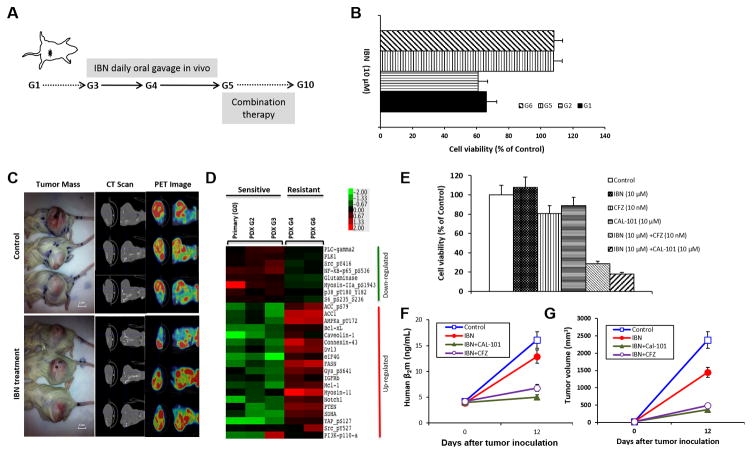
Figure 5. The identification of treatment combinations to overcome ibrutinib resistance.
A, Ibrutinib-naïve lymphoma cells were freshly isolated from apheresis of PT1-MCL. After PDX-G1 was established, the PDXs were passaged to next generations (G). Beginning in G3, the mice were treated with IBN (25 mg/kg, oral gavage daily). In G5, the mice were administered combination therapy to overcome drug resistance. B, Lymphoma cells were freshly isolated from G1, G2, G5, and G6 PDX tumors. Cell viability was tested by CellTiter-Glo luminescent cell viability assay after 48-hour incubation with 10 μM IBN. Cell viability in G5 and G6 was much higher than in G1 and G2 (p < 0.01), indicating that the PDX acquired IBN resistance in G5. C, The gross tumor mass, CT scan, and PET image showed no difference of tumor burden between vehicle control and ibrutinib-treated mice (p > 0.05, n = 3), validating the acquired resistance to IBN in PDX-G5. D, RPPA data showed the up-regulation and down-regulation of lymphoma-associated signaling pathways in IBN-sensitive and IBN-resistant tumor samples. E, The freshly isolated MCL cells from the G5 tumor mass were incubated with 10 μM IBN, 10 μM Cal-101, 10 nM CFZ, or 10 μM IBN plus 10 μM Cal-101 or 10 nM CFZ for 48 hours. Cell viability was tested by CellTiter-Glo luminescent cell viability assay (Control vs IBN, CFZ, or Cal-101, p > 0.05; Control vs IBN+Cal-101 or IBN+CFZ, p < 0.01). F, Mice were administered with vehicle control, ibrutinib 25 mg/kg oral gavage daily, with/without CAL101 25 mg/kg oral gavage daily, or CFZ 5 mg/kg i.v. on Days 1 and 5. Mouse serum was collected from tail vein blood on Days 1 and 12 of G5 tumor inoculation. F. Human β2M was detected by ELISA for monitoring tumor burden (Control vs IBN, p = 0.25; Control vs IBN+Cal-101 or IBN+CFZ, p < 0.01). G, Tumor volumes were calculated for monitoring tumor burden (Control vs IBN, p = 0.25; Control vs IBN+Cal-101 or IBN+CFZ, p < 0.01). PT, patient; IBN, ibrutinib; CFZ, carfilzomib.