Figure 1.
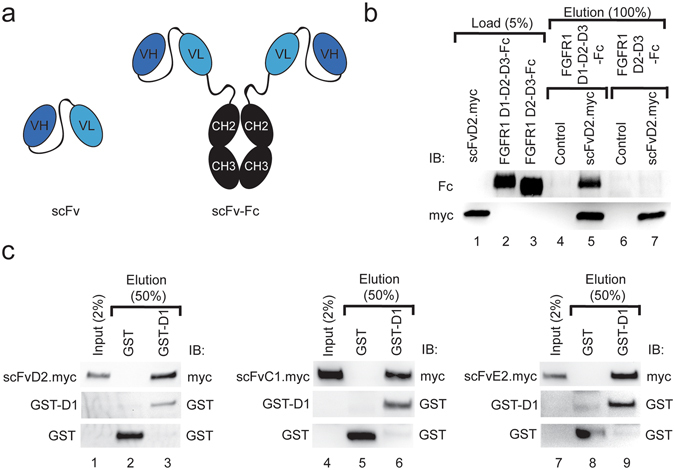
Antibody fragments bind to the domain D1 of FGFR1. (a) Schematic representation of anti-FGFR1 antibody fragments used in this study. The scFv antibody format contains single antigen binding site formed by variable domains of heavy and light antibody chains (VH and VL), whereas scFv-Fc antibody fragments contain two identical binding sites for antigen fused by constant domains of heavy chain of human IgG1 (CH2 and CH3). (b) Analyzed antibodies recognize epitopes within domain D1 of FGFR1. scFvD2.myc was bound to the anti-c-Myc agarose and incubated with either purified full length extracellular part of FGFR1 fused with Fc fragment (FGFR1 D1-D2-D3-Fc) or with the Fc-fusion of the extracellular part of FGFR1 lacking domain D1 (FGFR1 D2-D3-Fc). Proteins bound to scFvD2.myc were analyzed with anti-Fc antibodies. (c) Direct Interaction of scFv’s with the domain D1 of the FGFR1. Recombinant GST (Control) and GST-tagged domain D1 of the FGFR1 (GST-D1) were bound to Glutathione Sepharose and incubated with scFv proteins. Proteins bound to GST and GST-D1 were eluted and analyzed by Western blotting using specific antibodies. Cropped blots were displayed, full size blots are included in Supplementary Information.
