Figure 7.
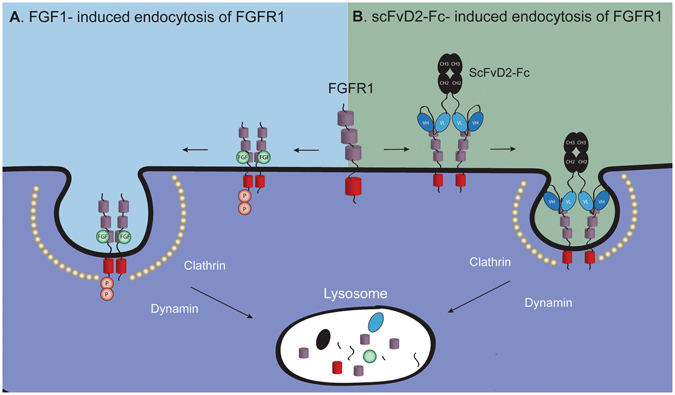
The model of the growth factor- and bivalent antibody-induced endocytosis of FGFR1. (a) FGF1 binds to the D2 and D3 domains of FGFR1 and induces FGFR1 dimerization. The conformational change within FGFR1 in the receptor dimer leads to the activation of FGFR1 kinase domain that results in receptor activation by transphosphorylation. Activated FGFR1 recruits intracellular signaling proteins and this results in the initiation of downstream signaling cascades. To downregulate the FGFR1-dependent signals FGFR1 dimer is sequestered into clathrin-coated vesicles and internalized into the cells via CME that requires dynamin. Subsequently FGFR1-containing endosomes are delivered for the lysosomal degradation. (b) The bivalent scFvD2-Fc antibody interacts with the D1 domain of FGFR1, leading to receptor dimerization. However, the conformation of the FGFR1 dimer is distinct from the one induced by FGF1, as there is no receptor activation. FGFR1 dimerization likely constitutes the signal for internalization of antibody-receptor complexes, as only bivalent antibody fragments are internalized. Next steps of scFvD2-Fc internalization, similarly to FGF1, employs FGFR1-mediated, clathrin and dynamin-dependent endocytosis. The scFvD2-Fc-FGFR1 complexes are finally degraded, likely in the lysosomes.
