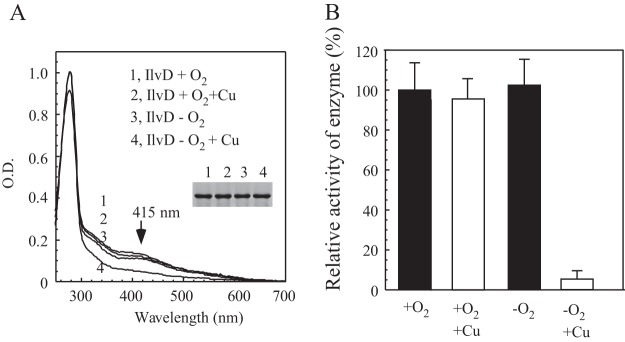
FIG 2.
Effect of copper on the iron-sulfur protein IlvD in E. coli cells under aerobic and anaerobic conditions. (A) UV-visible absorption spectra of recombinant IlvD purified from the E. coli cells grown in LB medium supplemented with or without 0.2 mM CuCl2 under aerobic or anaerobic conditions. Purified IlvD was dissolved in buffer containing 500 mM NaCl and 20 mM Tris (pH 8.0). The concentration of IlvD was 28 μM. (Inset) Photograph of SDS-PAGE gel of purified proteins. Samples: 1, IlvD under aerobic conditions; 2, IlvD under aerobic conditions with 0.2 mM CuCl2; 3, IlvD under anaerobic conditions; 4, IlvD under anaerobic conditions with 0.2 mM CuCl2. (B) Enzyme activity of IlvD. Recombinant IlvD was purified as described in panel A. For the enzyme activity assay, 1 μM IlvD was used. The highest enzyme activity (100%) for purified IlvD was 1.2 ± 0.1 mM/μM/min.