FIG 3.
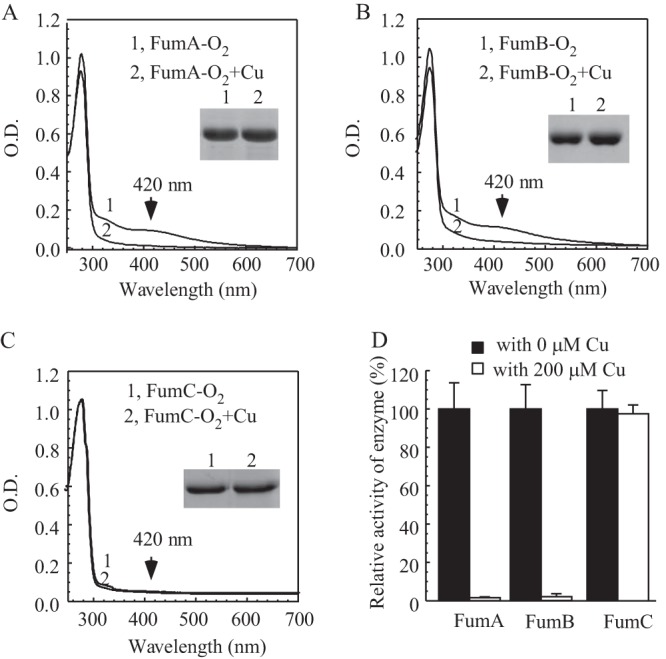
Effect of copper on fumarase A, B, and C in E. coli cells under anaerobic conditions. (A) UV-visible absorption spectra of recombinant fumarase A (20 μM) purified from E. coli cells grown in LB medium supplemented with 0 mM (spectrum 1) or 0.2 mM (spectrum 2) CuCl2 under anaerobic conditions. (B) UV-visible absorption spectra of recombinant fumarase B (20 μM) purified from E. coli cells grown in LB medium supplemented with 0 mM (spectrum 1) or 0.2 mM (spectrum 2) CuCl2 under anaerobic conditions. (C) UV-visible absorption spectra of recombinant fumarase C (30 μM) purified from E. coli cells grown in LB medium supplemented with 0 mM (spectrum 1) or 0.2 mM (spectrum 2) CuCl2 under anaerobic conditions. (Insets) Photograph of SDS-PAGE gels of purified proteins. (D) The enzyme activities of fumarases A, B, and C purified from E. coli cells grown in LB medium supplemented with 0 or 0.2 mM CuCl2 under anaerobic conditions. For the enzyme activity assay, 10 nM each fumarase was used. The highest enzyme activities (100%) for purified fumarase A, fumarase B, and fumarase C were 19.2 ± 0.3, 21.1 ± 0.1, and 16.6 ± 0.3 mM/μM/min, respectively. The results represent averages ± standard deviations from three independent experiments.
