Figure 5.
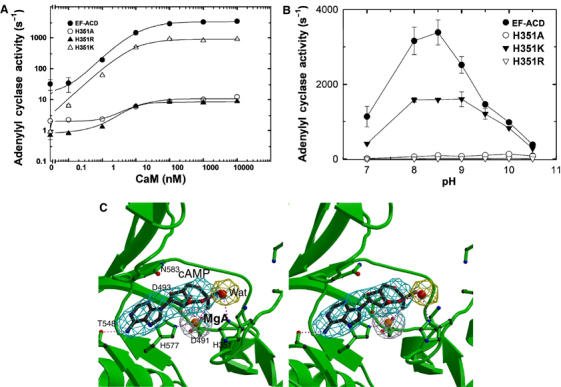
The catalytic mechanism of EF. (A) The adenylyl cyclase activity of wild-type EF-ACD and its mutants, H351A, H351K, and H351R (0.8 nM each) in response to the activation of CaM. The assay was performed at pH 7.2 in the presence of 10 mM MgCl2, 1 mM EDTA, 1 μM free CaCl2, 10 μM CaM, and 10 mM ATP. (B) The adenylyl cyclase activity of wild-type EF-ACD and its mutants H351A, H351K, and H351R in response to the pH changes. Adenylyl cyclase activity was measured in the presence of 10 mM ATP, 10 μM CaM, 1.2 μM free CaCl2, 500 μM BAPTA, and 10 mM MgCl2. Mean±s.e. are representative of at least two experiments. (C) The active site of EF in complex with CaM and cAMP. The simulated annealing omit map was contoured at the 3.0σ level. Oxygen, nitrogen, carbon, and metal atoms are in red, blue, black, and orange, respectively. Secondary structures of EF and ligands are in green and black, respectively.
